Abnormal brain development in child and adolescent carriers of mutant huntingtin
- PMID: 31371571
- PMCID: PMC6745731
- DOI: 10.1212/WNL.0000000000008066
Abnormal brain development in child and adolescent carriers of mutant huntingtin
Abstract
Objective: The huntingtin gene is critical for the formation and differentiation of the CNS, which raises questions about the neurodevelopmental effect of CAG expansion mutations within this gene (mHTT) that cause Huntington disease (HD). We sought to test the hypothesis that child and adolescent carriers of mHTT exhibit different brain growth compared to peers without the mutation by conducting structural MRI in youth who are at risk for HD. We also explored whether the length of CAG expansion affects brain development.
Methods: Children and adolescents (age 6-18) with a parent or grandparent diagnosed with HD underwent MRI and blinded genetic testing to confirm the presence or absence of mHTT. Seventy-five individuals were gene-expanded (GE) and 97 individuals were gene-nonexpanded (GNE). The GE group was estimated to be on average 35 years from clinical onset. Following an accelerated longitudinal design, age-related changes in brain regions were estimated.
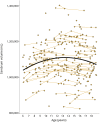
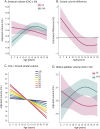
Results: Age-related striatal volume changes differed significantly between the GE and GNE groups, with initial hypertrophy and more rapid volume decline in GE. This pattern was exaggerated with CAG expansion length for CAG > 50. A similar age-dependent group difference was observed for the globus pallidus, but not in other major regions.
Conclusion: Our results suggest that pathogenesis of HD begins with abnormal brain development. An understanding of potential neurodevelopmental features associated with mHTT may be needed for optimized implementation of preventative gene silencing therapies, such that normal aspects of neurodevelopment are preserved as neurodegeneration is forestalled.
© 2019 American Academy of Neurology.
Figures


Comment on
-
Huntington disease: When does it begin?Neurology. 2019 Sep 3;93(10):421-422. doi: 10.1212/WNL.0000000000008054. Epub 2019 Aug 1. Neurology. 2019. PMID: 31371566 No abstract available.
References
-
- Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol 2011;10:83–98. - PubMed
-
- Cattaneo E, Zuccato C, Tartari M. Normal huntingtin function: an alternative approach to Huntington's disease. Nat Rev Neurosci 2005;6:919–930. - PubMed
-
- Godin JD, Colombo K, Molina-Calavita M, et al. . Huntingtin is required for mitotic spindle orientation and mammalian neurogenesis. Neuron 2010;67:392–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
