Identification of a neurocircuit underlying regulation of feeding by stress-related emotional responses
- PMID: 31371721
- PMCID: PMC6671997
- DOI: 10.1038/s41467-019-11399-z
Identification of a neurocircuit underlying regulation of feeding by stress-related emotional responses
Abstract
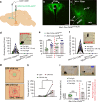
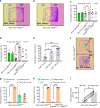
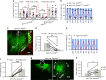
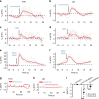
Feeding is known to be profoundly affected by stress-related emotional states and eating disorders are comorbid with psychiatric symptoms and altered emotional responses. The neural basis underlying feeding regulation by stress-related emotional changes is poorly understood. Here, we identify a novel projection from the paraventricular hypothalamus (PVH) to the ventral lateral septum (LSv) that shows a scalable regulation on feeding and behavioral changes related to emotion. Weak photostimulation of glutamatergic PVH→LSv terminals elicits stress-related self-grooming and strong photostimulation causes fear-related escape jumping associated with respective weak and strong inhibition on feeding. In contrast, inhibition of glutamatergic inputs to LSv increases feeding with signs of reduced anxiety. LSv-projecting neurons are concentrated in rostral PVH. LSv and LSv-projecting PVH neurons are activated by stressors in vivo, whereas feeding bouts were associated with reduced activity of these neurons. Thus, PVH→LSv neurotransmission underlies dynamic feeding by orchestrating emotional states, providing a novel neural circuit substrate underlying comorbidity between eating abnormalities and psychiatric disorders.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Defensive Behaviors Driven by a Hypothalamic-Ventral Midbrain Circuit.eNeuro. 2019 Jul 29;6(4):ENEURO.0156-19.2019. doi: 10.1523/ENEURO.0156-19.2019. Print 2019 Jul/Aug. eNeuro. 2019. PMID: 31331938 Free PMC article.
-
A neural basis for antagonistic control of feeding and compulsive behaviors.Nat Commun. 2018 Jan 4;9(1):52. doi: 10.1038/s41467-017-02534-9. Nat Commun. 2018. PMID: 29302029 Free PMC article.
-
Lateral septum as a melanocortin downstream site in obesity development.Cell Rep. 2023 May 30;42(5):112502. doi: 10.1016/j.celrep.2023.112502. Epub 2023 May 11. Cell Rep. 2023. PMID: 37171957 Free PMC article.
-
The Paraventricular Nucleus of the Hypothalamus: Development, Function, and Human Diseases.Endocrinology. 2018 Sep 1;159(9):3458-3472. doi: 10.1210/en.2018-00453. Endocrinology. 2018. PMID: 30052854 Review.
-
Neural network interactions and ingestive behavior control during anorexia.Physiol Behav. 2007 Jul 24;91(4):389-96. doi: 10.1016/j.physbeh.2007.04.010. Epub 2007 Apr 14. Physiol Behav. 2007. PMID: 17531275 Free PMC article. Review.
Cited by
-
Disrupted hypothalamic CRH neuron responsiveness contributes to diet-induced obesity.EMBO Rep. 2020 Jul 3;21(7):e49210. doi: 10.15252/embr.201949210. Epub 2020 May 27. EMBO Rep. 2020. PMID: 32462726 Free PMC article.
-
Acute and chronic stress differentially regulate pain via distinct ensembles in the paraventricular nucleus of hypothalamus.Mol Psychiatry. 2025 Aug 8. doi: 10.1038/s41380-025-03144-4. Online ahead of print. Mol Psychiatry. 2025. PMID: 40781546
-
A limbic circuitry involved in emotional stress-induced grooming.Nat Commun. 2020 May 8;11(1):2261. doi: 10.1038/s41467-020-16203-x. Nat Commun. 2020. PMID: 32385304 Free PMC article.
-
Dysfunctions of the paraventricular hypothalamic nucleus induce hypersomnia in mice.Elife. 2021 Nov 17;10:e69909. doi: 10.7554/eLife.69909. Elife. 2021. PMID: 34787078 Free PMC article.
-
Hypothalamic corticotropin-releasing hormone neurons modulate sevoflurane anesthesia and the post-anesthesia stress responses.Elife. 2024 Nov 11;12:RP90191. doi: 10.7554/eLife.90191. Elife. 2024. PMID: 39526880 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

