Cyfip1 haploinsufficient rats show white matter changes, myelin thinning, abnormal oligodendrocytes and behavioural inflexibility
- PMID: 31371763
- PMCID: PMC6671959
- DOI: 10.1038/s41467-019-11119-7
Cyfip1 haploinsufficient rats show white matter changes, myelin thinning, abnormal oligodendrocytes and behavioural inflexibility
Abstract
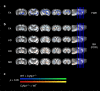
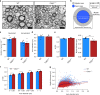
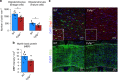
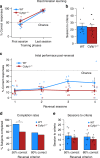
The biological basis of the increased risk for psychiatric disorders seen in 15q11.2 copy number deletion is unknown. Previous work has shown disturbances in white matter tracts in human carriers of the deletion. Here, in a novel rat model, we recapitulated low dosage of the candidate risk gene CYFIP1 present within the 15q11.2 interval. Using diffusion tensor imaging, we first showed extensive white matter changes in Cyfip1 mutant rats, which were most pronounced in the corpus callosum and external capsule. Transmission electron microscopy showed that these changes were associated with thinning of the myelin sheath in the corpus callosum. Myelin thinning was independent of changes in axon number or diameter but was associated with effects on mature oligodendrocytes, including aberrant intracellular distribution of myelin basic protein. Finally, we demonstrated effects on cognitive phenotypes sensitive to both disruptions in myelin and callosal circuitry.
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Damaged wires.Nat Rev Neurosci. 2019 Oct;20(10):575. doi: 10.1038/s41583-019-0216-3. Nat Rev Neurosci. 2019. PMID: 31455891 No abstract available.
-
Is There a Hemispheric Disconnect in Neurodevelopmental Disorders?Trends Neurosci. 2019 Dec;42(12):843-844. doi: 10.1016/j.tins.2019.10.005. Epub 2019 Nov 6. Trends Neurosci. 2019. PMID: 31704178
Similar articles
-
Mice with 16p11.2 Deletion and Duplication Show Alterations in Biological Processes Associated with White Matter.Int J Mol Sci. 2025 Jan 11;26(2):573. doi: 10.3390/ijms26020573. Int J Mol Sci. 2025. PMID: 39859289 Free PMC article.
-
Myelin loss and oligodendrocyte pathology in white matter tracts following traumatic brain injury in the rat.Eur J Neurosci. 2013 Jul;38(1):2153-65. doi: 10.1111/ejn.12179. Epub 2013 Mar 5. Eur J Neurosci. 2013. PMID: 23458840
-
The autism- and schizophrenia-associated protein CYFIP1 regulates bilateral brain connectivity and behaviour.Nat Commun. 2019 Aug 1;10(1):3454. doi: 10.1038/s41467-019-11203-y. Nat Commun. 2019. PMID: 31371726 Free PMC article.
-
White matter lactate--does it matter?Neuroscience. 2014 Sep 12;276:109-16. doi: 10.1016/j.neuroscience.2013.10.002. Epub 2013 Oct 12. Neuroscience. 2014. PMID: 24125892 Review.
-
White matter involvement after TBI: Clues to axon and myelin repair capacity.Exp Neurol. 2016 Jan;275 Pt 3:328-333. doi: 10.1016/j.expneurol.2015.02.011. Epub 2015 Feb 16. Exp Neurol. 2016. PMID: 25697845 Review.
Cited by
-
Oligodendrocyte-Specific Mechanisms of Myelin Thinning: Implications for Neurodegenerative Diseases.Front Neurosci. 2021 Mar 24;15:663053. doi: 10.3389/fnins.2021.663053. eCollection 2021. Front Neurosci. 2021. PMID: 33841096 Free PMC article. No abstract available.
-
Cytoplasmic FMR1 interacting protein (CYFIP) family members and their function in neural development and disorders.Mol Biol Rep. 2021 Aug;48(8):6131-6143. doi: 10.1007/s11033-021-06585-6. Epub 2021 Jul 29. Mol Biol Rep. 2021. PMID: 34327661 Review.
-
Cell-autonomous reduction of CYFIP2 is insufficient to induce Alzheimer's disease-like pathologies in the hippocampal CA1 pyramidal neurons of aged mice.Anim Cells Syst (Seoul). 2023 Mar 24;27(1):93-101. doi: 10.1080/19768354.2023.2192263. eCollection 2023. Anim Cells Syst (Seoul). 2023. PMID: 36999135 Free PMC article.
-
Structural and Lipidomic Alterations of Striatal Myelin in 16p11.2 Deletion Mouse Model of Autism Spectrum Disorder.Front Cell Neurosci. 2021 Aug 12;15:718720. doi: 10.3389/fncel.2021.718720. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34483844 Free PMC article.
-
Microglia regulate central nervous system myelin growth and integrity.Nature. 2023 Jan;613(7942):120-129. doi: 10.1038/s41586-022-05534-y. Epub 2022 Dec 14. Nature. 2023. PMID: 36517604 Free PMC article.
References
-
- Chai J-H, et al. Identification of four highly conserved genes between breakpoint hotspots BP1 and BP2 of the prader-willi/angelman syndromes deletion region that have undergone evolutionary transposition mediated by flanking duplicons. Am. J. Hum. Genet. 2003;73:898–925. doi: 10.1086/378816. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

