Aspergillus oryzae spore germination is enhanced by non-thermal atmospheric pressure plasma
- PMID: 31371801
- PMCID: PMC6673704
- DOI: 10.1038/s41598-019-47705-4
Aspergillus oryzae spore germination is enhanced by non-thermal atmospheric pressure plasma
Abstract
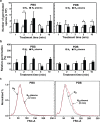
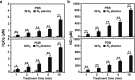
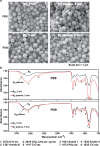
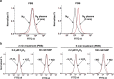
Poor and unstable culture growth following isolation presents a technical barrier to the efficient application of beneficial microorganisms in the food industry. Non-thermal atmospheric pressure plasma is an effective tool that could overcome this barrier. The objective of this study was to investigate the potential of plasma to enhance spore germination, the initial step in fungal colonization, using Aspergillus oryzae, a beneficial filamentous fungus used in the fermentation industry. Treating fungal spores in background solutions of phosphate buffered saline (PBS) and potato dextrose broth (PDB) with micro dielectric barrier discharge plasma using nitrogen gas for 2 and 5 min, respectively, significantly increased the germination percentage. Spore swelling, the first step in germination, was accelerated following plasma treatment, indicating that plasma may be involved in loosening the spore surface. Plasma treatment depolarized spore membranes, elevated intracellular Ca2+ levels, and activated mpkA, a MAP kinase, and the transcription of several germination-associated genes. Our results suggest that plasma enhances fungal spore germination by stimulating spore swelling, depolarizing the cell membrane, and activating calcium and MAPK signaling.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Vivek N, et al. Recent advances in the production of value added chemicals and lipids utilizing biodiesel industry generated crude glycerol as a substrate - Metabolic aspects, challenges and possibilities: An overview. Bioresour. Technol. 2017;239:507–517. doi: 10.1016/j.biortech.2017.05.056. - DOI - PubMed
-
- Feng R, Chen L, Chen K. Fermentation trip: amazing microbes, amazing metabolisms. Ann. Microbiol. 2018;68:717–729. doi: 10.1007/s13213-018-1384-5. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

