Bioceramics: from bone substitutes to nanoparticles for drug delivery
- PMID: 31371837
- PMCID: PMC6675605
- DOI: 10.1515/pac-2018-0505
Bioceramics: from bone substitutes to nanoparticles for drug delivery
Abstract
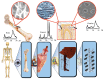
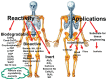
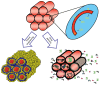
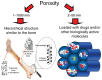
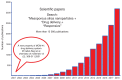
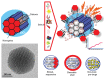
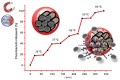
Since the second half of the 20th century, bioceramics are used for bone repair and regeneration. Inspired by bones and teeth, and aimed at mimicking their structure and composition, several artificial bioceramics were developed for biomedical applications. And nowadays, in the 21st century, with the increasing prominence of nanoscience and nanotechnology, certain bioceramics are being used to build smart drug delivery systems, among other applications. This minireview will mainly describe both tendencies through the research work carried out by the research team of María Vallet-Regí.
Keywords: Distinguished Women in Chemistry and Chemical Engineering; biomaterials; biomedical applications; ceramics; drug delivery.
Figures




Similar articles
-
Promising trends of bioceramics in the biomaterials field.J Mater Sci Mater Med. 2009 Feb;20(2):447-55. doi: 10.1007/s10856-008-3616-x. Epub 2008 Nov 6. J Mater Sci Mater Med. 2009. PMID: 18987955 Review.
-
Mesoporous Bioglasses Enriched with Bioactive Agents for Bone Repair, with a Special Highlight of María Vallet-Regí's Contribution.Pharmaceutics. 2022 Jan 15;14(1):202. doi: 10.3390/pharmaceutics14010202. Pharmaceutics. 2022. PMID: 35057097 Free PMC article. Review.
-
Bioceramics of calcium orthophosphates.Biomaterials. 2010 Mar;31(7):1465-85. doi: 10.1016/j.biomaterials.2009.11.050. Epub 2009 Dec 7. Biomaterials. 2010. PMID: 19969343 Review.
-
Additively manufactured Bi-functionalized bioceramics for reconstruction of bone tumor defects.Acta Biomater. 2023 Jan 15;156:234-249. doi: 10.1016/j.actbio.2022.08.042. Epub 2022 Aug 24. Acta Biomater. 2023. PMID: 36028198 Review.
-
Calcium Orthophosphate-Based Bioceramics.Materials (Basel). 2013 Sep 6;6(9):3840-3942. doi: 10.3390/ma6093840. Materials (Basel). 2013. PMID: 28788309 Free PMC article. Review.
Cited by
-
Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing.Int J Mol Sci. 2024 Jul 19;25(14):7914. doi: 10.3390/ijms25147914. Int J Mol Sci. 2024. PMID: 39063156 Free PMC article. Review.
-
Bioactive Materials for Soft Tissue Repair.Front Bioeng Biotechnol. 2021 Feb 19;9:613787. doi: 10.3389/fbioe.2021.613787. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33681157 Free PMC article. Review.
-
Factors influencing the drug release from calcium phosphate cements.Bioact Mater. 2021 May 30;7:341-363. doi: 10.1016/j.bioactmat.2021.05.032. eCollection 2022 Jan. Bioact Mater. 2021. PMID: 34466737 Free PMC article. Review.
-
QbD-Driven preparation, characterization, and pharmacokinetic investigation of daidzein-l oaded nano-cargos of hydroxyapatite.Sci Rep. 2025 Jan 23;15(1):2967. doi: 10.1038/s41598-025-85463-8. Sci Rep. 2025. PMID: 39848966 Free PMC article.
-
Advances in Biodegradable Polymers and Biomaterials for Medical Applications-A Review.Molecules. 2023 Aug 24;28(17):6213. doi: 10.3390/molecules28176213. Molecules. 2023. PMID: 37687042 Free PMC article. Review.
References
-
- Vallet-Regí M. Bioceramics with Clinical Applications. John Wiley & Sons; Chichester: 2014.
-
- Arcos D, Boccaccini AR, Bohner M, Díez-Pérez A, Epple M, Gómez-Barrena E, Herrera A, Planell JA, Rodríguez-Mañas L, Vallet-Regí M. Acta Biomater. 2014;10:1793. - PubMed
-
- Vallet-Regí M. In: A Report on the Biomaterials Research Translation in Europe. Vallet-Regí M, Missirlis YF, editors. European Society for Biomaterials; 2017. pp. 52–54. Opinion Leaders Papers.
-
- Vallet-Regí M, Salinas AJ. In: Bone Repair Biomaterials. Planell JA, Best SM, Lacroix D, Merolli A, editors. Woodhead Publishing Limited; Boca Raton: 2009. pp. 194–230.
-
- Vallet-Regí M. J Chem Soc Dalton Trans. 2001;97
Grants and funding
LinkOut - more resources
Full Text Sources
