From drug-delivery device to disease management tool: a study of preferences for enhanced features in next-generation self-injection devices
- PMID: 31371927
- PMCID: PMC6636455
- DOI: 10.2147/PPA.S203775
From drug-delivery device to disease management tool: a study of preferences for enhanced features in next-generation self-injection devices
Abstract
Purpose: To quantify rheumatology patient preferences and willingness to pay (WTP) for features differentiating enhanced from standard self-injection devices and to investigate differences among subgroups.
Patients and methods: Patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA), and axial spondyloarthritis (axSpA) were recruited in the UK. A discrete-choice experiment was used to elicit preferences; respondents were presented with 10 choices between 3 different devices: a free standard disposable device, and 2 hypothetical reusable devices characterized by presence/absence of skin sensor, injection speed control, on-screen instructions, injection reminders, electronic log, and large grip. Every hypothetical device included a cost component to assess WTP for each enhanced feature. A random-parameters logit model was used to estimate preference weights and WTP.
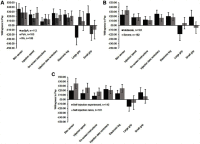
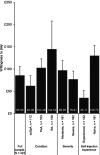
Results: Data were collected from 323 respondents by electronic survey (15/11/2017-15/02/2018; RA: 108; PsA: 103; axSpA: 112). On average, the skin sensor was the most preferred feature (£30), followed by injection speed control, injection reminders, electronic log (~£20 each), on-screen instructions (~£12), and a device with a small, rather than large grip (~£7). Similar preferences for attributes were observed across condition subgroups except for grip size: axSpA patients preferred small grip (~£27); PsA patients preferred large grip (~£19). Overall, respondents preferred reusable devices with all enhanced features (WTP value: £85) over the standard device. RA patients exhibited a higher WTP (£145) than PsA (£102) or axSpA (£62) for the same enhanced device.
Conclusion: Patients positively valued reusable self-injection devices with enhanced features, which may improve patient experience, potentially improving treatment adherence, clinical, and economic outcomes.
Keywords: discrete-choice experiment; patient preference; rheumatology; self-administration; subcutaneous injection.
Conflict of interest statement
MB: employee of RTI; BS: employee of UCB Pharma; BH: employee of RTI; BM: employee of RTI; IM: employee of UCB Pharma; MS: consultancy fees from UCB Pharma; NM: consultancy fees from UCB Pharma. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
Patient Preference for Self-Injection Devices in Rheumatoid Arthritis: A Discrete Choice Experiment in China.Patient Prefer Adherence. 2022 Aug 31;16:2387-2398. doi: 10.2147/PPA.S375938. eCollection 2022. Patient Prefer Adherence. 2022. PMID: 36068820 Free PMC article.
-
The Adherence and Outcomes Benefits of Using a Connected, Reusable Auto-Injector for Self-Injecting Biologics: A Narrative Review.Adv Ther. 2023 Nov;40(11):4758-4776. doi: 10.1007/s12325-023-02671-2. Epub 2023 Sep 21. Adv Ther. 2023. PMID: 37733212 Free PMC article. Review.
-
Six months multicentre pilot open label single-arm study to evaluate patient experience, acceptability and satisfaction of switching certolizumab pegol from a prefilled syringe or autoinjection pen to an AVA® e-Device in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis patients.J Clin Pharm Ther. 2022 Dec;47(12):2345-2349. doi: 10.1111/jcpt.13820. Epub 2022 Dec 5. J Clin Pharm Ther. 2022. PMID: 36470844
-
Enhancement of an Auto-Injector Device for Self-Administration of Etanercept in Patients With Rheumatoid Arthritis Confers Emotional and Functional Benefits.Rheumatol Ther. 2020 Sep;7(3):537-552. doi: 10.1007/s40744-020-00216-5. Epub 2020 Jun 4. Rheumatol Ther. 2020. PMID: 32500508 Free PMC article.
-
A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience.Drug Deliv. 2019 Dec;26(1):384-392. doi: 10.1080/10717544.2019.1587043. Drug Deliv. 2019. PMID: 30905213 Free PMC article. Review.
Cited by
-
Patient Preference for Self-Injection Devices in Rheumatoid Arthritis: A Discrete Choice Experiment in China.Patient Prefer Adherence. 2022 Aug 31;16:2387-2398. doi: 10.2147/PPA.S375938. eCollection 2022. Patient Prefer Adherence. 2022. PMID: 36068820 Free PMC article.
-
The Adherence and Outcomes Benefits of Using a Connected, Reusable Auto-Injector for Self-Injecting Biologics: A Narrative Review.Adv Ther. 2023 Nov;40(11):4758-4776. doi: 10.1007/s12325-023-02671-2. Epub 2023 Sep 21. Adv Ther. 2023. PMID: 37733212 Free PMC article. Review.
-
Digital Health Integration Assessment and Maturity of the United States Biopharmaceutical Industry: Forces Driving the Next Generation of Connected Autoinjectable Devices.JMIR Mhealth Uhealth. 2021 Mar 18;9(3):e25406. doi: 10.2196/25406. JMIR Mhealth Uhealth. 2021. PMID: 33621188 Free PMC article.
-
Single-Injection Options for Administering a 320 mg Dose of Bimekizumab: 2 mL Safety Syringe and Auto-injector.Dermatol Ther (Heidelb). 2025 May;15(5):1113-1134. doi: 10.1007/s13555-025-01366-6. Epub 2025 Mar 29. Dermatol Ther (Heidelb). 2025. PMID: 40156698 Free PMC article.
-
The Evolving Landscape of Discrete Choice Experiments in Health Economics: A Systematic Review.Pharmacoeconomics. 2025 Aug;43(8):879-936. doi: 10.1007/s40273-025-01495-y. Epub 2025 May 21. Pharmacoeconomics. 2025. PMID: 40397369 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

