NIR-guided dendritic nanoplatform for improving antitumor efficacy by combining chemo-phototherapy
- PMID: 31371941
- PMCID: PMC6635674
- DOI: 10.2147/IJN.S203171
NIR-guided dendritic nanoplatform for improving antitumor efficacy by combining chemo-phototherapy
Abstract
Background: Phototherapy, including photothermal therapy (PTT) and photodynamic therapy (PDT), is a promising noninvasive strategy in the treatment of cancers due to its highly localized specificity to tumors and minimal side effects to normal tissues. However, single phototherapy often causes tumor recurrence which hinders its clinical applications. Therefore, developing a NIR-guided dendritic nanoplatform for improving the phototherapy effect and reducing the recurrence of tumors by synergistic chemotherapy and phototherapy is essential.
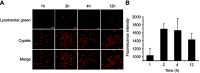
Methods: A fluorescent targeting ligand, insisting of ICG derivative cypate and a tumor penetration peptide iRGD (CRGDKGPDC), was covalently combined with PAMAM dendrimer to prepare a single agent-based dendritic theranostic nanoplatform iRGD-cypate-PAMAM-DTX (RCPD).
Results: Compared with free cypate, the resulted RCPD could generate enhanced singlet oxygen species while maintaining its fluorescence intensity and heat generation ability when subjected to NIR irradiation. Furthermore, our in vitro and in vivo therapeutic studies demonstrated that compared with phototherapy or chemotherapy alone, the combinatorial chemo-photo treatment of RCPD with the local exposure of NIR light can significantly improve anti-tumor efficiency and reduce the risk of recurrence of tumors.
Conclusion: The multifunctional theranostic platform (RCPD) could be used as a promising method for NIR fluorescence image-guided combinatorial treatment of tumor cancers.
Keywords: ICG derivatives; chemotherapy; combined therapy; dendrimer; phototherapy.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






Similar articles
-
Dendrimer-encapsulated naphthalocyanine as a single agent-based theranostic nanoplatform for near-infrared fluorescence imaging and combinatorial anticancer phototherapy.Nanoscale. 2015 Mar 7;7(9):3888-902. doi: 10.1039/c4nr06050d. Nanoscale. 2015. PMID: 25422147
-
Tumor-targeted and multi-stimuli responsive drug delivery system for near-infrared light induced chemo-phototherapy and photoacoustic tomography.Acta Biomater. 2016 Jul 1;38:129-42. doi: 10.1016/j.actbio.2016.04.024. Epub 2016 Apr 16. Acta Biomater. 2016. PMID: 27090593
-
Development of a Highly Efficient NIR-II Phototherapeutic Agent for Fluorescence Imaging-Guided Synergistic PTT/PDT/Chemotherapy of Colorectal Cancer.J Med Chem. 2025 Apr 10;68(7):7592-7604. doi: 10.1021/acs.jmedchem.5c00066. Epub 2025 Apr 1. J Med Chem. 2025. PMID: 40168043
-
NIR-activated multifunctional agents for the combined application in cancer imaging and therapy.Adv Colloid Interface Sci. 2025 Feb;336:103356. doi: 10.1016/j.cis.2024.103356. Epub 2024 Nov 22. Adv Colloid Interface Sci. 2025. PMID: 39612723 Review.
-
Recent Progress in Carrier-Free Nanomedicine for Tumor Phototherapy.Adv Healthc Mater. 2023 Feb;12(4):e2202307. doi: 10.1002/adhm.202202307. Epub 2022 Nov 24. Adv Healthc Mater. 2023. PMID: 36349844 Review.
Cited by
-
Chemosensitivity enhanced by autophagy inhibition based on a polycationic nano-drug carrier.Nanoscale Adv. 2021 Jan 27;3(6):1656-1673. doi: 10.1039/d0na00990c. eCollection 2021 Mar 23. Nanoscale Adv. 2021. PMID: 36132550 Free PMC article.
-
Extracellular vesicles: a rising star for therapeutics and drug delivery.J Nanobiotechnology. 2023 Jul 20;21(1):231. doi: 10.1186/s12951-023-01973-5. J Nanobiotechnology. 2023. PMID: 37475025 Free PMC article. Review.
-
Black phosphorus nanosheets and docetaxel micelles co-incorporated thermoreversible hydrogel for combination chemo-photodynamic therapy.Drug Deliv Transl Res. 2021 Jun;11(3):1133-1143. doi: 10.1007/s13346-020-00836-y. Drug Deliv Transl Res. 2021. PMID: 32776211
-
Self-assembly of Peptide dendrimers and their bio-applications in theranostics.Mater Today Bio. 2022 Mar 8;14:100239. doi: 10.1016/j.mtbio.2022.100239. eCollection 2022 Mar. Mater Today Bio. 2022. PMID: 35295319 Free PMC article.
-
Mechanism Investigation of Hyaluronidase-Combined Multistage Nanoparticles for Solid Tumor Penetration and Antitumor Effect.Int J Nanomedicine. 2020 Aug 24;15:6311-6324. doi: 10.2147/IJN.S257164. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32922003 Free PMC article.
References
-
- Song X, Chen Q, Liu Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015;8(2):340–354. doi:10.1007/s12274-014-0620-y - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

