A case of chronic eosinophilic pneumonia in a patient treated with dupilumab
- PMID: 31371974
- PMCID: PMC6636310
- DOI: 10.2147/TCRM.S207402
A case of chronic eosinophilic pneumonia in a patient treated with dupilumab
Abstract
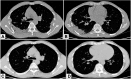
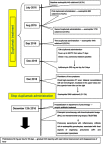
The increasing knowledge on inflammatory pathways has driven the development of targeted biological therapies for severe refractory asthma. Among the recently developed biologics, the fully human monoclonal antibody dupilumab is an interesting therapeutic option, given its ability to inhibit the biological effects of both IL-4 and IL-13. We describe the case of a male, Caucasian, 56-year-old patient with allergic and eosinophilic severe asthma. Given the poor asthma control, he started treatment with add-on dupilumab, and after the tenth injection, he presented with a fever and bilateral pulmonary thickening. A significant increase in blood eosinophilia was also reported. The patient underwent a fiberoptic bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial lung biopsy (TBLB/TBB). BAL revealed eosinophils alveolitis (60%) while TBB showed findings compatible with chronic eosinophilic pneumonia (CEP). After prolonged treatment with oral corticosteroids, the clinical picture improved with resolution of CEP. Since the beginning of dupilumab treatment, simultaneously to a great improvement in asthma control, the patient showed a progressive increase in blood eosinophils count and subsequent onset of clinical-radiological pattern suggestive of CEP. Based on published data, dupilumab may have induced an alteration of the complex immunological pathway of our patient. This pathway is affected by both allergic and eosinophilic asthmatic endotypes, and consequently, the concomitant action of allergenic stimuli and eosinophils may have caused the appearance of eosinophilic pneumonia. To our knowledge, this is the first reported case of CEP as a possible severe side effect of dupilumab administration.
Keywords: dupilumab; eosinophilic pneumonia; eosinophils; fiberoptic bronchoscopy; monoclonal antibodies; severe asthma.
Conflict of interest statement
Francesco Menzella participated in contracted research and clinical trials for Novartis and Sanofi; has received lecture fees and advisory board fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma and Novartis. Nicola Facciolongo served as a consultant for Boston Scientific and has received lecture fees from AstraZeneca and Chiesi. The other authors report no conflicts of interest in this work.
Figures


References
-
- Loza MJ, Djukanovic R, Chung KF, et al. ADEPT (Airways disease endotyping for personalized therapeutics) and U-BIOPRED (Unbiased biomarkers for the prediction of respiratory disease outcome consortium) investigators. Validated and longitudinally stable asthma phenotypes based on cluster analysis of the ADEPT study. Respir Res. 2016;17(1):165. - PMC - PubMed
LinkOut - more resources
Full Text Sources

