The immunomodulatory-drug, lenalidomide, sustains and enhances interferon-α production by human plasmacytoid dendritic cells
- PMID: 31372079
- PMCID: PMC6635835
- DOI: 10.2147/JBM.S206459
The immunomodulatory-drug, lenalidomide, sustains and enhances interferon-α production by human plasmacytoid dendritic cells
Abstract
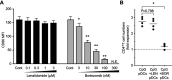
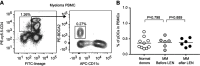
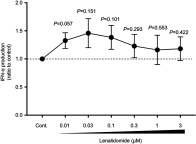
Background: Lenalidomide (LEN), an immunomodulatory drug (IMiD), is currently used for treatment of multiple myeloma (MM). LEN potentiates T cell and natural killer cell functions. However, the cellular and molecular mechanisms underlying the immunomodulatory effects of LEN remain unclear. We focused on the effects of LEN on human plasmacytoid dendritic cells (pDCs), which are the major source of interferon (IFN)-α in the blood and play a central role in innate immune responses. Results: We found that bortezomib, a proteasome inhibitor used to treat MM, killed pDCs but that 0.1-3 μM LEN (covering clinical plasma concentration range) did not affect pDC survival or CD86 expression. Bortezomib inhibited pDC-derived IFN-α production in a dose-dependent fashion, but 0.1-3 µM LEN sustained pDC-derived IFN-α production when stimulated with an optimal concentration of CpG-ODN 2216 (3 μM). In pDCs stimulated with a low concentration of CpG-ODN (0.1 μM), LEN enhanced IFN-α production. These results indicated that LEN, when used at a clinically relevant concentration, can potentially enhance IFN-α production by pDCs. Conclusion: Collectively, our findings unveiled a novel target of LEN and extend the repertoire of the drug's known immunomodulatory effects. These effects may explain the low incidence of herpes zoster viral infection observed during LEN treatment compared with bortezomib treatment. LEN may function as an IMiD affecting a wide array of immune cells, including pDCs, leading to amplification of a positive immune axis able to eliminate MM cells.
Keywords: IMiDs; lenalidomide; multiple myeloma; plasmacyotid DCs; type I IFNs.
Conflict of interest statement
Tomoki Ito received honoraria from Celgene, Bristol-Myers Squibb, and Takeda. Tomoki Ito also reports personal fees from Celgene, Bristol-Myers Squibb, and Takeda, during the conduct of the study. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
Distinctive downmodulation of plasmacytoid dendritic cell functions by vitamin D3 analogue calcipotriol.J Dermatol Sci. 2016 Oct;84(1):71-79. doi: 10.1016/j.jdermsci.2016.06.003. Epub 2016 Jun 3. J Dermatol Sci. 2016. PMID: 27342039
-
HMGB1 Is Involved in IFN-α Production and TRAIL Expression by HIV-1-Exposed Plasmacytoid Dendritic Cells: Impact of the Crosstalk with NK Cells.PLoS Pathog. 2016 Feb 12;12(2):e1005407. doi: 10.1371/journal.ppat.1005407. eCollection 2016 Feb. PLoS Pathog. 2016. PMID: 26871575 Free PMC article.
-
Lenalidomide increases human dendritic cell maturation in multiple myeloma patients targeting monocyte differentiation and modulating mesenchymal stromal cell inhibitory properties.Oncotarget. 2017 May 23;8(32):53053-53067. doi: 10.18632/oncotarget.18085. eCollection 2017 Aug 8. Oncotarget. 2017. PMID: 28881793 Free PMC article.
-
Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells.Eur J Immunol. 2001 Jul;31(7):2154-63. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. Eur J Immunol. 2001. PMID: 11449369
-
Lenalidomide in Multiple Myeloma: Review of Resistance Mechanisms, Current Treatment Strategies and Future Perspectives.Cancers (Basel). 2023 Feb 2;15(3):963. doi: 10.3390/cancers15030963. Cancers (Basel). 2023. PMID: 36765919 Free PMC article. Review.
Cited by
-
Expression and correlation of IL-2, IL-10 and TNF-α in patients with multiple myeloma-infected herpes zoster treated by bortezomib-containing regimen.Am J Transl Res. 2021 Dec 15;13(12):13732-13740. eCollection 2021. Am J Transl Res. 2021. PMID: 35035711 Free PMC article.
-
Immunomodulatory drugs suppress Th1-inducing ability of dendritic cells but enhance Th2-mediated allergic responses.Blood Adv. 2020 Aug 11;4(15):3572-3585. doi: 10.1182/bloodadvances.2019001410. Blood Adv. 2020. PMID: 32761232 Free PMC article.
-
Thalidomide augments maturation and T helper 1-inducing capacity of monocyte-derived dendritic cells in vitro.Bioimpacts. 2024 Dec 29;15:30588. doi: 10.34172/bi.30588. eCollection 2025. Bioimpacts. 2024. PMID: 40256218 Free PMC article.
-
A Case Report of a 58-Year-Old Woman with a Diagnosis of High-Risk Myeloma Refractory to Multiple Line of Therapy and Treated with Selinexor, Bortezomib, and Dexamethasone Prior to Allogeneic Stem Cell Transplantation.Am J Case Rep. 2022 Apr 21;23:e935353. doi: 10.12659/AJCR.935353. Am J Case Rep. 2022. PMID: 35444159 Free PMC article.
-
Reactivation of Hepatitis B Virus in Patients with Multiple Myeloma.Cancers (Basel). 2019 Nov 19;11(11):1819. doi: 10.3390/cancers11111819. Cancers (Basel). 2019. PMID: 31752356 Free PMC article. Review.
References
-
- Gandhi AK, Kang J, Capone L, et al. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Curr Cancer Drug Targets. 2010;10(2):155–167. - PubMed
LinkOut - more resources
Full Text Sources

