A hydroponics based high throughput screening system for Phytophthora root rot resistance in chickpea (Cicer arietinum L.)
- PMID: 31372178
- PMCID: PMC6659211
- DOI: 10.1186/s13007-019-0463-3
A hydroponics based high throughput screening system for Phytophthora root rot resistance in chickpea (Cicer arietinum L.)
Abstract
Background: Phytophthora root rot (PRR) caused by P. medicaginis is a major soil borne disease in chickpea growing regions of Australia. Sources of resistance have been identified in both cultivated and wild Cicer species. However, the molecular basis underlying PRR resistance is not known. Current phenotyping methods rely on mycelium slurry or oospore inoculum. Sensitive and reliable methods are desirable to study variation for PRR resistance in chickpea and allow for a controlled inoculation process to better capture early defence responses following PRR infection.
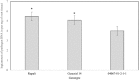
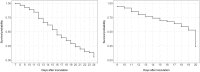
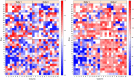
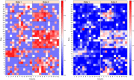
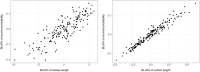
Results: In this study, a procedure for P. medicaginis zoospore production was standardized and used as the inoculum to develop a hydroponics based in planta infection method to screen chickpea genotypes with established levels of PRR resistance. The efficiency of the system was both qualitatively validated based on observation of characteristic PRR symptom development, and quantitatively validated based on the amount of pathogen DNA in roots. This system was scaled up to screen two biparental mapping populations previously developed for PRR studies. For each of the screenings, plant survival time was measured after inoculation and used to derive Kaplan-Meier estimates of plant survival (KME-survival). KME-survival and canker length were then selected as phenotypic traits associated with PRR resistance. Genetic analysis of these traits was conducted which identified quantitative trait loci (QTL). Additionally, these hydroponic traits and a set of previously published plant survival traits obtained from multiple PRR field experiments were combined in a model-based correlation analysis. The results suggest that the underlying genetic basis for plant survival during PRR infection within hydroponics and field disease environments is linked. The QTL QRBprrkms03 and QRBprrck03 on chromosome 4 identified for the traits KME-survival and canker length, respectively, correspond to the same region reported for PRR resistance in a field disease experiment.
Conclusion: A hydroponics based screening system will facilitate reliable and rapid screening in both small- and large-scale experiments to study PRR disease in chickpea. It can be applied in chickpea breeding programs to screen for PRR resistance and classify the virulence of new and existing P. medicaginis isolates.
Keywords: Combined hydroponics and field trait model; Hydroponics screening system; Kaplan–Meier (KM) estimates of survival probability; Linear mixed model; P. medicaginis zoospores production; PRR phenotyping method; PRR resistance in chickpea; Plant survival traits; Whole genome QTL analysis.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures

References
-
- Knights EJ, Açıkgöz N, Warkentin T, Bejiga G, Yadav SS, Sandhu JS. Area, production and distribution. In: Yadav RJR SS, Chen W, Sharma B, editors. Chickpea breeding and management. Wallingford: CABI; 2007. pp. 167–178.
-
- Yadav SS, Chen W. Chickpea breeding and management. Wallingford: CABI; 2007.
-
- Knights EJ, Southwell RJ, Schwinghamer MW, Harden S. Resistance to Phytophthora medicaginis Hansen and Maxwell in wild Cicer species and its use in breeding root rot resistant chickpea (Cicer arietinum L.) Aust J Agric Res. 2008;59:383–387. doi: 10.1071/AR07175. - DOI
-
- Murray GMBJ. The current and potential costs from diseases of oilseed crops in Australia. ACT, Australia: GRDC; 2012.
-
- Irwin J, Cahill D, Drenth A. Phytophthora in Australia. Aust J Agric Res. 1995;46:1311–1337. doi: 10.1071/AR9951311. - DOI
LinkOut - more resources
Full Text Sources

