Biodegradable crosslinked polyesters derived from thiomalic acid and S-nitrosothiol analogues for nitric oxide release
- PMID: 31372219
- PMCID: PMC6675467
- DOI: 10.1039/C8TB00566D
Biodegradable crosslinked polyesters derived from thiomalic acid and S-nitrosothiol analogues for nitric oxide release
Abstract
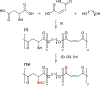
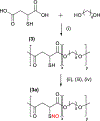
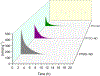
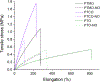
Crosslinked polyesters with Young's moduli similar to that of certain soft biological tissues were prepared via bulk polycondensation of thiomalic acid and 1,8-octanediol alone, and with citric or maleic acid. The copolymers were converted to nitric oxide (NO)-releasing S-nitrosothiol (RSNO) analogues by reaction with tert-butyl nitrite. Additional conjugation steps were avoided by inclusion of the thiolated monomer during the polycondensation to permit thiol conversion to RSNOs. NO release at physiological pH and temperature (pH 7.4, 37 °C) was determined by chemiluminescence-based NO detection. The average total NO content for poly(thiomalic-co-maleic acid-co-1,8-octanediol), poly(thiomalic-co-citric acid-co-1,8-octanediol), and poly(thiomalic acid-co-1,8-octanediol) was 130 ± 39 μmol g-1, 200 ± 35 μmol g-1, and 130 ± 11 μmol g-1, respectively. The antibacterial properties of the S-nitrosated analogues were confirmed against Escherichia coli and Staphylococcus aureus. The hydrolytic degradation products were analyzed by time-of-flight mass spectrometry after a 10-week study to investigate their composition. Tensile mechanical tests were performed on the non-nitrosated polymers as well as their S-nitrosated derivatives and suggested that the materials have appropriate Young's moduli and elongation values for biomedical applications.
Conflict of interest statement
Conflicts of interest There are no conflicts to declare.
Figures
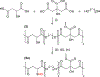

References
-
- Bettinger CJ, Pure Appl. Chem, 2010, 83, 9–24.
-
- Dhand C, Venkatesh M, Barathi VA, Harini S, Bairagi S, Goh Tze Leng E, Muruganandham N, Low KZW, Fazil MHUT, Loh XJ, Srinivasan DK, Liu SP, Beuerman RW, Verma NK, Ramakrishna S and Lakshminarayanan R, Biomaterials, 2017, 138, 153–168. - PubMed
-
- Hubbell JA, Nat. Biotechnol, 1995, 13, 565.
-
- Nair LS and Laurencin CT, Prog. Polym. Sci, 2007, 32, 762–798.
-
- Li S, J. Biomed. Mater. Res, 1999, 48, 342–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

