Gastric dysmotility in Parkinson's disease is not caused by alterations of the gastric pacemaker cells
- PMID: 31372495
- PMCID: PMC6659650
- DOI: 10.1038/s41531-019-0087-3
Gastric dysmotility in Parkinson's disease is not caused by alterations of the gastric pacemaker cells
Abstract
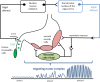
The enteric nervous system is involved in the pathology of Parkinson´s disease and patients frequently have symptoms related to delayed gastric emptying. However, the pathophysiology of gastric dysmotility is yet not well understood. The objective of this study was to assess interdigestive gastric motility in Parkinson´s disease. Using an electromagnetic capsule system, the dominant gastric contraction frequency (primary outcome measure) and the gastric transit time were assessed in 16 patients with Parkinson´s disease and 15 young healthy controls after a fasting period of 8 h. Motor and non-motor symptoms were assessed using the Movement Disorder Society Unified Parkinson´s Disease Rating Scale III (MDS-UPDRS III), the Non-Motor Symptoms Questionnaire (NMS-Quest), and Hoehn & Yahr staging. The Gastroparesis Cardinal Symptom Index was used to record symptoms related to delayed gastric emptying. In healthy controls and patients with Parkinson's disease, the dominant contraction frequency was 3.0 cpm indicating normal function of interstitial cells of Cajal. In patients with Parkinson's disease, the gastric transit time was longer than in younger controls (56 vs. 21 min). The dominant contraction frequency and gastric transit time did not correlate with age, disease duration, Hoehn & Yahr stage, levodopa equivalent daily dose, MDS-UPDRS III, NMS-Quest, and Gastroparesis Cardinal Symptom Index. Changes of gastric motility in Parkinson´s disease are not caused by functional deficits of the gastric pacemaker cells, the interstitial cells of Cajal. Therefore, gastroparesis in Parkinson's disease can be attributed to disturbances in neurohumoral signals via the vagus nerve and myenteric plexus.
Keywords: Parkinson's disease; Physiology.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

