Targeting of lipid metabolism with a metabolic inhibitor cocktail eradicates peritoneal metastases in ovarian cancer cells
- PMID: 31372520
- PMCID: PMC6668395
- DOI: 10.1038/s42003-019-0508-1
Targeting of lipid metabolism with a metabolic inhibitor cocktail eradicates peritoneal metastases in ovarian cancer cells
Erratum in
-
Author Correction: Targeting of lipid metabolism with a metabolic inhibitor cocktail eradicates peritoneal metastases in ovarian cancer cells.Commun Biol. 2025 Feb 23;8(1):294. doi: 10.1038/s42003-025-07638-3. Commun Biol. 2025. PMID: 39988621 Free PMC article. No abstract available.
Abstract
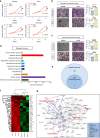
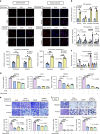
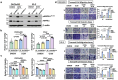
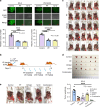
Ovarian cancer is an intra-abdominal tumor in which the presence of ascites facilitates metastatic dissemination, and associated with poor prognosis. However, the significance of metabolic alterations in ovarian cancer cells in the ascites microenvironment remains unclear. Here we show ovarian cancer cells exhibited increased aggressiveness in ascites microenvironment via reprogramming of lipid metabolism. High lipid metabolic activities are found in ovarian cancer cells when cultured in the ascites microenvironment, indicating a metabolic shift from aerobic glycolysis to β-oxidation and lipogenesis. The reduced AMP-activated protein kinase (AMPK) activity due to the feedback effect of high energy production led to the activation of its downstream signaling, which in turn, enhanced the cancer growth. The combined treatment of low toxic AMPK activators, the transforming growth factor beta-activated kinase 1 (TAK1) and fatty acid synthase (FASN) inhibitors synergistically impair oncogenic augmentation of ovarian cancer. Collectively, targeting lipid metabolism signaling axis impede ovarian cancer peritoneal metastases.
Keywords: Cancer metabolism.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures



References
-
- Roett, M. A. & Evans, P. Ovarian cancer: an overview. Am. Fam. Physician80, 609–616 (2009). - PubMed
-
- Bristow, R. E., Tomacruz, R. S., Armstrong, D. K., Trimble, E. L. & Montz, F. J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J. Clin. Oncol.20, 1248–1259 (2002). - PubMed
-
- Vergote, I. et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New Engl. J. Med.363, 943–953 (2010). - PubMed
-
- Khan, S. M. et al. In vitro metastatic colonization of human ovarian cancer cells to the omentum. Clin. Exp. Metastas.27, 185–196 (2010). - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

