Systematic review of methodology used in clinical studies evaluating the benefits of proton beam therapy
- PMID: 31372521
- PMCID: PMC6660607
- DOI: 10.1016/j.ctro.2019.07.002
Systematic review of methodology used in clinical studies evaluating the benefits of proton beam therapy
Abstract
Background: Proton beam therapy (PBT) delivers high-energy radiation to target tumours while sparing surrounding normal tissues. The dosimetric advantages of PBT over traditional photon radiotherapy may be clear but the translation of this benefit into clinically meaningful reductions in toxicities and improved quality-of-life (QoL) needs to be determined. Randomised controlled trials (RCTs) are considered the gold standard for generating the highest-level evidence in medicine. The objectives of this systematic review were to provide an overview of published clinical studies evaluating the benefits of PBT, and to examine the methodology used in clinical trials with respect to study design and outcomes.
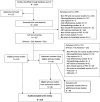
Methods: PubMed, EMBASE and Cochrane databases were systematically searched for published clinical studies where PBT was a cancer treatment intervention. All randomised and non-randomised studies, prospective or retrospective, were eligible for inclusion.
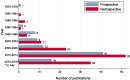
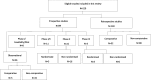
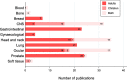
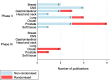
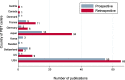
Results: In total, 219 studies were included. Prospective studies comprised 89/219 (41%), and of these, the number of randomised phase II and III trials were 5/89 (6%) and 3/89 (3%) respectively. Of all the phase II and III trials, 18/24 (75%) were conducted at a single PBT centre. Over one-third of authors recommended an increase in length of follow up. Research design and/or findings were poorly reported in 74/89 (83%) of prospective studies. Patient reported outcomes were assessed in only 19/89 (21%) of prospective studies.
Conclusions: Prospective randomised evidence for PBT is limited. The set-up of national PBT services in several countries provides an opportunity to guide the optimal design of prospective studies, including RCTs, to evaluate the benefits of PBT across various disease sites. Collaboration between PBT centres, both nationally and internationally, would increase potential for the generation of practice changing evidence. There is a need to facilitate and guide the collection and analysis of meaningful outcome data, including late toxicities and patient reported QoL.
Keywords: Clinical trial methodology; Patient reported outcomes; Proton beam therapy; Randomized controlled trials; Systematic review.
Figures
Similar articles
-
Hitting the Target: Developing High-quality Evidence for Proton Beam Therapy Through Randomised Controlled Trials.Clin Oncol (R Coll Radiol). 2024 Feb;36(2):70-79. doi: 10.1016/j.clon.2023.11.027. Epub 2023 Nov 8. Clin Oncol (R Coll Radiol). 2024. PMID: 38042671 Review.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
The Promise of Proton Beam Therapy for Oesophageal Cancer: A Systematic Review of Dosimetric and Clinical Outcomes.Clin Oncol (R Coll Radiol). 2021 Aug;33(8):e339-e358. doi: 10.1016/j.clon.2021.04.003. Epub 2021 Apr 28. Clin Oncol (R Coll Radiol). 2021. PMID: 33931290
-
Hadrontherapy for cancer. An overview of HTA reports and ongoing studies.Recenti Prog Med. 2019 Dec;110(12):566-586. doi: 10.1701/3278.32516. Recenti Prog Med. 2019. PMID: 31909760 Review. English.
-
Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: a systematic review.J Gastrointest Oncol. 2016 Aug;7(4):644-64. doi: 10.21037/jgo.2016.05.06. J Gastrointest Oncol. 2016. PMID: 27563457 Free PMC article.
Cited by
-
Boron Nanoparticle-Enhanced Proton Therapy: Molecular Mechanisms of Tumor Cell Sensitization.Molecules. 2024 Aug 21;29(16):3936. doi: 10.3390/molecules29163936. Molecules. 2024. PMID: 39203014 Free PMC article.
-
Selecting patients for proton beam therapy.J Med Radiat Sci. 2021 Mar;68(1):2-3. doi: 10.1002/jmrs.454. Epub 2020 Dec 1. J Med Radiat Sci. 2021. PMID: 33259660 Free PMC article.
-
Harnessing benefit of highly conformal RT techniques for lymphoma patients.Br J Radiol. 2021 Nov 1;94(1127):20210469. doi: 10.1259/bjr.20210469. Epub 2021 Aug 11. Br J Radiol. 2021. PMID: 34379521 Free PMC article. Review.
-
Stereotactic or Conventional Radiation for Early-Stage Non-small Cell Lung Cancer: A Systematic Review and Meta-Analysis.Cureus. 2023 Apr 27;15(4):e38198. doi: 10.7759/cureus.38198. eCollection 2023 Apr. Cureus. 2023. PMID: 37252503 Free PMC article. Review.
-
TORPEdO: A phase III trial of intensity-modulated proton beam therapy versus intensity-modulated radiotherapy for multi-toxicity reduction in oropharyngeal cancer.Clin Transl Radiat Oncol. 2022 Nov 21;38:147-154. doi: 10.1016/j.ctro.2022.11.010. eCollection 2023 Jan. Clin Transl Radiat Oncol. 2022. PMID: 36452431 Free PMC article.
References
-
- Fuss M., Hug E.B., Schaefer R.A. Proton radiation therapy (prt) for pediatric optic pathway gliomas: comparison with 3d planned conventional photons and a standard photon technique. Int J Radiat Oncol Biol Phys. 1999;45(5):1117–1126. - PubMed
-
- Archambeau J.O., Slater J.D., Slater J.M., Tangeman R. Role for proton beam irradiation in treatment of pediatric cns malignancies. Int J Radiat Oncol Biol Phys. 1992;22(2):287–294. - PubMed
-
- Lin R., Hug E.B., Schaefer R.A., Miller D.W., Slater J.M., Slater J.D. Conformal proton radiation therapy of the posterior fossa: a study comparing protons with three-dimensional planned photons in limiting dose to auditory structures. Int J Radiat Oncol Biol Phys. 2000;48(4):1219–1226. - PubMed
-
- Yock T., Schneider R., Friedmann A., Adams J., Fullerton B., Tarbell N. Proton radiotherapy for orbital rhabdomyosarcoma: clinical outcome and a dosimetric comparison with photons. Int J Radiat Oncol Biol Phys. 2005;63(4):1161–1168. - PubMed
-
- MacDonald S.M., Safai S., Trofimov A. Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys. 2008;71(4):979–986. - PubMed
LinkOut - more resources
Full Text Sources

