Knowledge and use of recruitment support tools among study coordinators at an academic medical center: The Novel Approaches to Recruitment Planning Study
- PMID: 31372576
- PMCID: PMC6661275
- DOI: 10.1016/j.conctc.2019.100424
Knowledge and use of recruitment support tools among study coordinators at an academic medical center: The Novel Approaches to Recruitment Planning Study
Abstract
Background: Study coordinators play an essential role on study teams; however, there remains a paucity of research on the supports and services they need to effectively recruit and retain study participants.
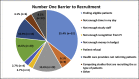
Methods: A cross-sectional survey was conducted with 147 study coordinators from a large academic medical center. Survey items assessed barriers and facilitators to recruitment and retention, anxiety about reaching enrollment numbers, confidence for talking to potential study participants about research involvement, awareness and use of CTSA resources, and PI involvement with recruitment planning.
Results: Significant associations were found between anxiety about reaching target enrollment numbers and whether the study coordinator was the primary person responsible for developing a recruitment strategy. Three years or more serving as a study coordinator and levels of anxiety for reaching enrollment numbers was also significant.
Conclusion: More institutional level supports and formal training opportunities are needed to enhance study coordinators' effectiveness to recruit participants.
Keywords: Academic medical centers; Clinical trials as topic; Cross-sectional studies; National center for advancing translational sciences (U.S.); Patient selection; Research personnel.
Figures
References
-
- Speicher L.A., Fromell G., Avery S. The critical need for academic health centers to assess the training, support, and career development requirements of clinical research coordinators: recommendations from the clinical and translational science award research coordinator taskforce. Clin. Transl. Sci. 2012;5(6):470–475. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous