Transdermal estradiol for postpartum depression: results from a pilot randomized, double-blind, placebo-controlled study
- PMID: 31372757
- PMCID: PMC10105981
- DOI: 10.1007/s00737-019-00991-3
Transdermal estradiol for postpartum depression: results from a pilot randomized, double-blind, placebo-controlled study
Abstract
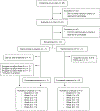
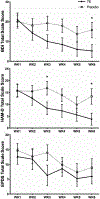
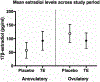
Postpartum depression (PPD) is a common complication following delivery, though evidence-based treatment options are limited. This study explores the feasibility and efficacy of outpatient PPD treatment with transdermal estradiol (TE). In a pilot, double-blind, placebo-controlled trial, women with PPD were randomized to receive transdermal 17β-estradiol (100 mcg/day) or placebo patch. Over 6 weeks, women completed weekly ratings on the Beck Depression Inventory (BDI), Edinburgh Postnatal Depression Scale (EPDS), and Hamilton Depression Scale (HAM-D). Primary outcome measures were treatment response (> 50% decrease from baseline BDI) and remission (BDI < 10) at 6 weeks, and secondary outcome measures included severity on all scales at weeks 3 and 6. Of 12 recruited women, 6 received TE and 6 received placebo. By week 6, 5 women receiving TE responded to treatment and 4 showed symptom remission, compared to 2 responders and 1 remitter in the placebo group. This difference was not significant (p = 0.24). In a mixed-model of BDI ratings, TE was associated with a 9.2 point decrease at 3 weeks (95%CI - 19.5 to + 1.0, p = 0.074) and a 10.5 point decrease at 6 weeks (95%CI - 21.0-0.0, p = 0.049) compared to placebo, though these differences did not survive multiple comparisons correction. Analogous effects were found for HAM-D but not EPDS scores. Interestingly, no significant difference in plasma estradiol levels existed between groups. We were unable to demonstrate a significant therapeutic benefit of TE compared with placebo in PPD. Although limited by under-recruitment and loss to follow-up, our results suggest TE is a feasible option for outpatient PPD management, with preliminary evidence (based on secondary outcomes) for efficacy. Therapeutic effects may be seen as early as 3 weeks and may not directly depend on peripheral measures of estradiol.
Keywords: Postpartum depression; Randomized controlled trial; Transdermal estradiol.
Conflict of interest statement
Figures
Similar articles
-
The short-term effects of estradiol, raloxifene, and a phytoestrogen in women with perimenopausal depression.Menopause. 2021 Jan 15;28(4):369-383. doi: 10.1097/GME.0000000000001724. Menopause. 2021. PMID: 33470755 Free PMC article. Clinical Trial.
-
Effects of Estradiol Withdrawal on Mood in Women With Past Perimenopausal Depression: A Randomized Clinical Trial.JAMA Psychiatry. 2015 Jul;72(7):714-26. doi: 10.1001/jamapsychiatry.2015.0111. JAMA Psychiatry. 2015. PMID: 26018333 Free PMC article. Clinical Trial.
-
Transdermal oestrogen for treatment of severe postnatal depression.Lancet. 1996 Apr 6;347(9006):930-3. doi: 10.1016/s0140-6736(96)91414-2. Lancet. 1996. PMID: 8598756 Clinical Trial.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
Transdermal estradiol for postpartum depression: a promising treatment option.Clin Obstet Gynecol. 2009 Sep;52(3):516-29. doi: 10.1097/GRF.0b013e3181b5a395. Clin Obstet Gynecol. 2009. PMID: 19661765 Free PMC article. Review.
Cited by
-
Comparative efficacy and acceptability of pharmacotherapies for postpartum depression: A systematic review and network meta-analysis.Front Pharmacol. 2022 Nov 24;13:950004. doi: 10.3389/fphar.2022.950004. eCollection 2022. Front Pharmacol. 2022. PMID: 36506537 Free PMC article.
-
The link between sex hormones and depression over a woman's lifespan (Review).Biomed Rep. 2025 Feb 20;22(4):71. doi: 10.3892/br.2025.1949. eCollection 2025 Apr. Biomed Rep. 2025. PMID: 40083602 Free PMC article. Review.
-
Short-term oestrogen as a strategy to prevent postpartum depression in high-risk women: protocol for the double-blind, randomised, placebo-controlled MAMA clinical trial.BMJ Open. 2021 Dec 30;11(12):e052922. doi: 10.1136/bmjopen-2021-052922. BMJ Open. 2021. PMID: 35763351 Free PMC article.
-
The short-term effects of estradiol, raloxifene, and a phytoestrogen in women with perimenopausal depression.Menopause. 2021 Jan 15;28(4):369-383. doi: 10.1097/GME.0000000000001724. Menopause. 2021. PMID: 33470755 Free PMC article. Clinical Trial.
-
Progesterone, reproduction, and psychiatric illness.Best Pract Res Clin Obstet Gynaecol. 2020 Nov;69:108-126. doi: 10.1016/j.bpobgyn.2020.06.001. Epub 2020 Jun 18. Best Pract Res Clin Obstet Gynaecol. 2020. PMID: 32723604 Free PMC article. Review.
References
-
- Ahokas A, Kaukoranta J, Wahlbeck K, Aito M (2001) Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry 62:332–336 - PubMed
-
- American Psychiatric Association, American Psychiatric Association, Task Force on DSM-IV (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association, Washington, DC
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

