Biomechanical modeling of transcatheter aortic valve replacement in a stenotic bicuspid aortic valve: deployments and paravalvular leakage
- PMID: 31372826
- PMCID: PMC6801083
- DOI: 10.1007/s11517-019-02012-y
Biomechanical modeling of transcatheter aortic valve replacement in a stenotic bicuspid aortic valve: deployments and paravalvular leakage
Abstract
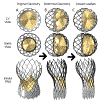
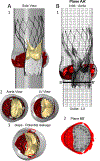
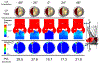
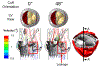
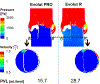
Calcific aortic valve disease (CAVD) is characterized by stiffened aortic valve leaflets. Bicuspid aortic valve (BAV) is the most common congenital heart disease. Transcatheter aortic valve replacement (TAVR) is a treatment approach for CAVD where a stent with mounted bioprosthetic valve is deployed on the stenotic valve. Performing TAVR in calcified BAV patients may be associated with post-procedural complications due to the BAV asymmetrical structure. This study aims to develop refined computational models simulating the deployments of Evolut R and PRO TAVR devices in a representative calcified BAV. The paravalvular leakage (PVL) was also calculated by computational fluid dynamics simulations. Computed tomography scan of severely stenotic BAV patient was acquired. The 3D calcium deposits were generated and embedded inside a parametric model of the BAV. Deployments of the Evolut R and PRO inside the calcified BAV were simulated in five bioprosthesis leaflet orientations. The hypothesis of asymmetric and elliptic stent deployment was confirmed. Positioning the bioprosthesis commissures aligned with the native commissures yielded the lowest PVL (15.7 vs. 29.5 mL/beat). The Evolut PRO reduced the PVL in half compared with the Evolut R (15.7 vs. 28.7 mL/beat). The proposed biomechanical computational model could optimize future TAVR treatment in BAV patients. Graphical abstract.
Keywords: Bicuspid aortic valve; Computational fluid dynamics; Finite element; Paravalvular leakage; Transcatheter aortic valve replacement.
Conflict of interest statement
Figures





Similar articles
-
Designing a Novel Asymmetric Transcatheter Aortic Valve for Stenotic Bicuspid Aortic Valves Using Patient-Specific Computational Modeling.Ann Biomed Eng. 2023 Jan;51(1):58-70. doi: 10.1007/s10439-022-03039-3. Epub 2022 Aug 30. Ann Biomed Eng. 2023. PMID: 36042099 Free PMC article.
-
Validating In Silico and In Vitro Patient-Specific Structural and Flow Models with Transcatheter Bicuspid Aortic Valve Replacement Procedure.Cardiovasc Eng Technol. 2022 Dec;13(6):840-856. doi: 10.1007/s13239-022-00620-8. Epub 2022 Apr 7. Cardiovasc Eng Technol. 2022. PMID: 35391657 Free PMC article.
-
A Bicuspid Aortic Valve Imaging Classification for the TAVR Era.JACC Cardiovasc Imaging. 2016 Oct;9(10):1145-1158. doi: 10.1016/j.jcmg.2015.12.022. Epub 2016 Jun 29. JACC Cardiovasc Imaging. 2016. PMID: 27372022
-
Transcatheter aortic valve replacement for bicuspid aortic valve stenosis with first- and new-generation bioprostheses: A systematic review and meta-analysis.Int J Cardiol. 2020 Jan 1;298:76-82. doi: 10.1016/j.ijcard.2019.09.003. Epub 2019 Sep 6. Int J Cardiol. 2020. PMID: 31575495
-
Transcatheter aortic valve replacement in bicuspid aortic valve stenosis.Prog Cardiovasc Dis. 2020 Jul-Aug;63(4):482-487. doi: 10.1016/j.pcad.2020.06.007. Epub 2020 Jun 25. Prog Cardiovasc Dis. 2020. PMID: 32592707 Review.
Cited by
-
Analysis of fibrocalcific aortic valve stenosis: computational pre-and-post TAVR haemodynamics behaviours.R Soc Open Sci. 2024 Feb 21;11(2):230905. doi: 10.1098/rsos.230905. eCollection 2024 Feb. R Soc Open Sci. 2024. PMID: 38384780 Free PMC article.
-
Pooled-Analysis of Association of Sievers Bicuspid Aortic Valve Morphology With New Permanent Pacemaker and Conduction Abnormalities After Transcatheter Aortic Valve Replacement.Front Cardiovasc Med. 2022 May 26;9:884911. doi: 10.3389/fcvm.2022.884911. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35694658 Free PMC article.
-
Simulation study of transcatheter heart valve implantation in patients with stenotic bicuspid aortic valve.Med Biol Eng Comput. 2020 Apr;58(4):815-829. doi: 10.1007/s11517-020-02138-4. Epub 2020 Feb 6. Med Biol Eng Comput. 2020. PMID: 32026185
-
Finite Element Analysis of Evolut Transcatheter Heart Valves: Effects of Aortic Geometries and Valve Sizes on Post-TAVI Wall Stresses and Deformations.J Clin Med. 2025 Jan 27;14(3):850. doi: 10.3390/jcm14030850. J Clin Med. 2025. PMID: 39941521 Free PMC article.
-
Designing a Novel Asymmetric Transcatheter Aortic Valve for Stenotic Bicuspid Aortic Valves Using Patient-Specific Computational Modeling.Ann Biomed Eng. 2023 Jan;51(1):58-70. doi: 10.1007/s10439-022-03039-3. Epub 2022 Aug 30. Ann Biomed Eng. 2023. PMID: 36042099 Free PMC article.
References
-
- Thubrikar M (1989) The Aortic Valve. CRC Press
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

