Molecular Mimicry, Autoimmunity, and Infection: The Cross-Reactive Antigens of Group A Streptococci and their Sequelae
- PMID: 31373269
- PMCID: PMC6684244
- DOI: 10.1128/microbiolspec.GPP3-0045-2018
Molecular Mimicry, Autoimmunity, and Infection: The Cross-Reactive Antigens of Group A Streptococci and their Sequelae
Abstract
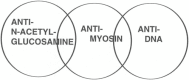
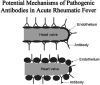
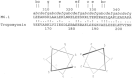
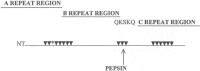
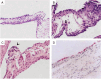
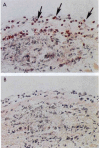
The group A streptococci are associated with a group of diseases affecting the heart, brain, and joints that are collectively referred to as acute rheumatic fever. The streptococcal immune-mediated sequelae, including acute rheumatic fever, are due to antibody and cellular immune responses that target antigens in the heart and brain as well as the group A streptococcal cross-reactive antigens as reviewed in this article. The pathogenesis of acute rheumatic fever, rheumatic heart disease, Sydenham chorea, and other autoimmune sequelae is related to autoantibodies that are characteristic of autoimmune diseases and result from the immune responses against group A streptococcal infection by the host. The sharing of host and streptococcal epitopes leads to molecular mimicry between the streptococcal and host antigens that are recognized by the autoantibodies during the host response. This article elaborates on the discoveries that led to a better understanding of the pathogenesis of disease and provides an overview of the history and the most current thought about the immune responses against the host and streptococcal cross-reactive antigens in group A streptococcal sequelae.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

