New insights into the development of the human cerebral cortex
- PMID: 31373394
- PMCID: PMC6704245
- DOI: 10.1111/joa.13055
New insights into the development of the human cerebral cortex
Abstract
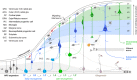
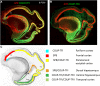
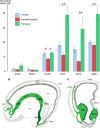
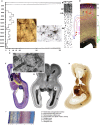
The cerebral cortex constitutes more than half the volume of the human brain and is presumed to be responsible for the neuronal computations underlying complex phenomena, such as perception, thought, language, attention, episodic memory and voluntary movement. Rodent models are extremely valuable for the investigation of brain development, but cannot provide insight into aspects that are unique or highly derived in humans. Many human psychiatric and neurological conditions have developmental origins but cannot be studied adequately in animal models. The human cerebral cortex has some unique genetic, molecular, cellular and anatomical features, which need to be further explored. The Anatomical Society devoted its summer meeting to the topic of Human Brain Development in June 2018 to tackle these important issues. The meeting was organized by Gavin Clowry (Newcastle University) and Zoltán Molnár (University of Oxford), and held at St John's College, Oxford. The participants provided a broad overview of the structure of the human brain in the context of scaling relationships across the brains of mammals, conserved principles and recent changes in the human lineage. Speakers considered how neuronal progenitors diversified in human to generate an increasing variety of cortical neurons. The formation of the earliest cortical circuits of the earliest generated neurons in the subplate was discussed together with their involvement in neurodevelopmental pathologies. Gene expression networks and susceptibility genes associated to neurodevelopmental diseases were discussed and compared with the networks that can be identified in organoids developed from induced pluripotent stem cells that recapitulate some aspects of in vivo development. New views were discussed on the specification of glutamatergic pyramidal and γ-aminobutyric acid (GABA)ergic interneurons. With the advancement of various in vivo imaging methods, the histopathological observations can be now linked to in vivo normal conditions and to various diseases. Our review gives a general evaluation of the exciting new developments in these areas. The human cortex has a much enlarged association cortex with greater interconnectivity of cortical areas with each other and with an expanded thalamus. The human cortex has relative enlargement of the upper layers, enhanced diversity and function of inhibitory interneurons and a highly expanded transient subplate layer during development. Here we highlight recent studies that address how these differences emerge during development focusing on diverse facets of our evolution.
Keywords: GABA; associative areas; calretinin; inhibitory interneurons; neurogenesis; neuroimaging; neuronal progenitors; prefrontal cortex; subplate neurons.
© 2019 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
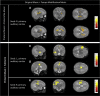
Figures



References
-
- Adam‐Darque A, Grouiller F, Vasung L, et al. (2017) fMRI‐based neuronal response to new odorants in the newborn brain. Cereb Cortex 27, 1–7. - PubMed
-
- Alfano C, Studer M (2013) Neocortical arealization: evolution, mechanisms, and open questions. Dev Neurobiol 73, 411–447. - PubMed
-
- Allendoerfer K, Shatz C (1994) The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci 17, 185–218. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS090029/NS/NINDS NIH HHS/United States
- R01 NS092339/NS/NINDS NIH HHS/United States
- BB/F003285/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R35 NS116859/NS/NINDS NIH HHS/United States
- G0700377/MRC_/Medical Research Council/United Kingdom
- R01 MH060929/MH/NIMH NIH HHS/United States
- G0300200/MRC_/Medical Research Council/United Kingdom
- R01 HD098657/HD/NICHD NIH HHS/United States
- G0900901/MRC_/Medical Research Council/United Kingdom
- MC_PC_15102/MRC_/Medical Research Council/United Kingdom
- MR/N026039/1/MRC_/Medical Research Council/United Kingdom
- G0500180/MRC_/Medical Research Council/United Kingdom

