Longitudinal non-adherence predicts treatment escalation in paediatric ulcerative colitis
- PMID: 31373712
- PMCID: PMC8215554
- DOI: 10.1111/apt.15445
Longitudinal non-adherence predicts treatment escalation in paediatric ulcerative colitis
Abstract
Background: Medication non-adherence in paediatric ulcerative colitis (UC) has been associated with negative health outcomes including flares in disease activity. However, no studies to date have examined longitudinal adherence to maintenance medication in a prospective controlled trial.
Aims: To determine whether objectively measured adherence to standardised mesalazine (mesalamine) therapy over time was related to remission at 52 weeks and the need for treatment escalation in newly diagnosed paediatric patients with UC METHODS: PROTECT (NCT01536535) was a prospective, inception cohort, multi-site study of paediatric patients aged 4-17 years with newly diagnosed UC followed for 52 weeks. Patients received standardised mesalazine, with pre-established criteria for escalation to thiopurines or anti-TNFα inhibitors. Patients used pill bottles with electronic caps to monitor mesalazine adherence. We tested whether longitudinal adherence to mesalazine predicted steroid-free remission at week 52 (i.e. quiescent disease on mesalazine alone with no corticosteroids ≥4 weeks prior) and need for treatment escalation (i.e. introduction of immunomodulators, calcineurin-inhibitors or anti-TNFα inhibitors).
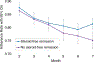
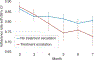
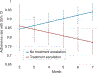
Results: Among 268 patients, average mesalazine adherence trajectories did not predict week 52 steroid-free remission. Declining adherence over time strongly predicted treatment escalation (β = -.037, P = .001). By month 6, adherence rate ≤85.7% was associated with treatment escalation.
Conclusions: Non-adherence may have affected therapeutic efficacy of standardised mesalazine, thereby contributing to need for treatment escalation. Routine adherence monitoring for at least 6 months following treatment initiation and addressing adherence difficulties early in the disease course are recommended.
Keywords: adherence trajectories; children; clinical remission; paediatric; treatment escalation; ulcerative colitis.
© 2019 John Wiley & Sons Ltd.
Figures

Similar articles
-
Factors associated with early outcomes following standardised therapy in children with ulcerative colitis (PROTECT): a multicentre inception cohort study.Lancet Gastroenterol Hepatol. 2017 Dec;2(12):855-868. doi: 10.1016/S2468-1253(17)30252-2. Epub 2017 Sep 20. Lancet Gastroenterol Hepatol. 2017. PMID: 28939374 Free PMC article.
-
Clinical and biological predictors of response to standardised paediatric colitis therapy (PROTECT): a multicentre inception cohort study.Lancet. 2019 Apr 27;393(10182):1708-1720. doi: 10.1016/S0140-6736(18)32592-3. Epub 2019 Mar 29. Lancet. 2019. PMID: 30935734 Free PMC article. Clinical Trial.
-
Evolution and predictive factors of relapse in ulcerative colitis patients treated with mesalazine after a first course of corticosteroids.J Crohns Colitis. 2011 Jun;5(3):196-202. doi: 10.1016/j.crohns.2010.12.011. Epub 2011 Feb 10. J Crohns Colitis. 2011. PMID: 21575881
-
A practical guide to the management of distal ulcerative colitis.Drugs. 1998 Apr;55(4):519-42. doi: 10.2165/00003495-199855040-00004. Drugs. 1998. PMID: 9561342 Review.
-
Mesalamine in the treatment and maintenance of remission of ulcerative colitis.Expert Rev Clin Pharmacol. 2012 Mar;5(2):113-23. doi: 10.1586/ecp.12.2. Expert Rev Clin Pharmacol. 2012. PMID: 22390554 Free PMC article. Review.
Cited by
-
Ethical considerations in using sensors to remotely assess pediatric health behaviors.Am Psychol. 2024 Jan;79(1):39-51. doi: 10.1037/amp0001196. Am Psychol. 2024. PMID: 38236214 Free PMC article.
-
What the Patient Thinks and What the Patient Does: Placebo, Nocebo, and Therapy Adherence in Ulcerative Colitis.J Clin Med. 2025 Jun 18;14(12):4351. doi: 10.3390/jcm14124351. J Clin Med. 2025. PMID: 40566096 Free PMC article. Review.
-
The Impact of COVID-19 on Pediatric Adherence and Self-Management.J Pediatr Psychol. 2020 Oct 1;45(9):977-982. doi: 10.1093/jpepsy/jsaa079. J Pediatr Psychol. 2020. PMID: 32929482 Free PMC article. Review.
-
A Micro-longitudinal Approach to Measuring Medication Adherence in Pediatric Inflammatory Bowel Diseases.J Pediatr Gastroenterol Nutr. 2020 Sep;71(3):366-370. doi: 10.1097/MPG.0000000000002778. J Pediatr Gastroenterol Nutr. 2020. PMID: 32404759 Free PMC article.
-
Fitness-for-use of Retrospective Multicenter Electronic Health Records to Conduct Outcome Analysis for Pediatric Ulcerative Colitis.Medicine (Baltimore). 2024 Mar 15;103(11):e37395. doi: 10.1097/MD.0000000000037395. Medicine (Baltimore). 2024. PMID: 38489703 Free PMC article.
References
-
- Higgins PD, Rubin D, Kaulback K, Schoenfield P, Kane S. Systematic review: impact of non-adherence to 5-aminosalicylic acid products on the frequency and cost of ulcerative colitis flares. Aliment Pharmacol Ther. 2009;29:247–257. - PubMed
-
- Oliva-Hemker MM, Abadom V, Cuffari C, Thompson RE. Nonadherence with thiopurine immunomodulator and mesalamine medications in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2007;44:180–184. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
