Amiodarone use and the risk of acute pancreatitis: Influence of different exposure definitions
- PMID: 31373736
- PMCID: PMC6916315
- DOI: 10.1002/pds.4851
Amiodarone use and the risk of acute pancreatitis: Influence of different exposure definitions
Abstract
Purpose: The antiarrhythmic drug amiodarone has a long half-life of 60 days, which is often ignored in observational studies. This study aimed to investigate the impact of different exposure definitions on the association between amiodarone use and the risk of acute pancreatitis.
Method: Using data from the Dutch PHARMO Database Network, incident amiodarone users were compared to incident users of a different type of antiarrhythmic drug. Eighteen different definitions were applied to define amiodarone exposure, including dichotomized, continuous and categorized cumulative definitions with lagged effects to account for the half-life of amiodarone. For each exposure definition, a Cox proportional hazards model was used to estimate the hazard ratio (HR) of hospitalization for acute pancreatitis.
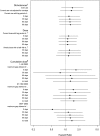
Results: This study included 15,378 starters of amiodarone and 21,394 starters of other antiarrhythmic drugs. Adjusted HRs for acute pancreatitis ranged between 1.21-1.43 for dichotomized definitions of exposure to amiodarone, between 1.13-1.22 for dose definitions (per DDD) and between 0.52-1.72 for cumulative dose definitions, depending on the category. Accounting for lagged effects had little impact on estimated HRs.
Conclusions: This study demonstrates the relative insensitivity of the association between amiodarone and the risk of acute pancreatitis against a broad range of different exposure definitions. Accounting for possible lagged effects had little impact, possibly because treatment switching and discontinuation was uncommon in this population.
Keywords: amiodarone; drug therapy; pancreatitis; pharmacoepidemiology; research design.
© 2019 The Authors. Pharmacoepidemiology & Drug Safety Published by John Wiley & Sons Ltd.
Conflict of interest statement
No authors report any conflict of interest, and there were no sponsors supporting this work.
Figures

References
-
- KNMP . Amiodaron In: Informatorium Medicamentorum. The Hague, The Netherlands; Knmp Medicijn Media; 2017.
-
- Freedman MD, Somberg JC. Pharmacology and pharmacokinetics of amiodarone. J Clin Pharmacol. 1991;31(11):1061‐1069. http://www.ncbi.nlm.nih.gov/pubmed/1753010 Accessed August 8, 2017 - PubMed
-
- Kashima A, Funahashi M, Fukumoto K, et al. Pharmacokinetic characteristics of amiodarone in long‐term oral therapy in Japanese population. Biol Pharm Bull. 2005;28(10):1934‐1938. http://www.ncbi.nlm.nih.gov/pubmed/16204949 Accessed August 8, 2017 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

