Ventricular remodeling in ischemic heart failure stratifies responders to stem cell therapy
- PMID: 31373782
- PMCID: PMC6954701
- DOI: 10.1002/sctm.19-0149
Ventricular remodeling in ischemic heart failure stratifies responders to stem cell therapy
Abstract
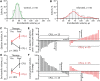
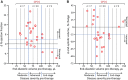
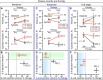
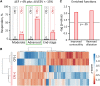
Response to stem cell therapy in heart failure is heterogeneous, warranting a better understanding of outcome predictors. This study assessed left ventricular volume, a surrogate of disease severity, on cell therapy benefit. Small to large infarctions were induced in murine hearts to model moderate, advanced, and end-stage ischemic cardiomyopathy. At 1 month postinfarction, cardiomyopathic cohorts with comparable left ventricular enlargement and dysfunction were randomized 1:1 to those that either received sham treatment or epicardial delivery of cardiopoietic stem cells (CP). Progressive dilation and pump failure consistently developed in sham. In comparison, CP treatment produced significant benefit at 1 month post-therapy, albeit with an efficacy impacted by cardiomyopathic stage. Advanced ischemic cardiomyopathy was the most responsive to CP-mediated salvage, exhibiting both structural and functional restitution, with proteome deconvolution substantiating that cell therapy reversed infarction-induced remodeling of functional pathways. Moderate cardiomyopathy was less responsive to CP therapy, improving contractility but without reversing preexistent heart enlargement. In end-stage disease, CP therapy showed the least benefit. This proof-of-concept study thus demonstrates an optimal window, or "Goldilocks principle," of left ventricular enlargement for maximized stem cell-based cardiac repair. Disease severity grading, prior to cell therapy, should be considered to inform regenerative medicine interventions.
Keywords: cardiopoiesis; left ventricular volume; myocardial infarction; outcome; proteomics; regenerative medicine.
© 2019 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
S.Y., A.B., and A.T. are coinventors on regenerative sciences related intellectual property disclosed to Mayo Clinic. Previously, Mayo Clinic has administered research grants from Celyad. Mayo Clinic, A.B., and A.T. have interests in Rion LLC.
Figures
References
-
- Normand C, Kaye DM, Povsic TJ, et al. Beyond pharmacological treatment: an insight into therapies that target specific aspects of heart failure pathophysiology. Lancet. 2019;393:1045‐1055. - PubMed
-
- Braunwald E. Cell‐based therapy in cardiac regeneration: an overview. Circ Res. 2018;123:132‐137. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

