Bayesian hypothesis testing and experimental design for two-photon imaging data
- PMID: 31374071
- PMCID: PMC6693774
- DOI: 10.1371/journal.pcbi.1007205
Bayesian hypothesis testing and experimental design for two-photon imaging data
Erratum in
-
Correction: Bayesian hypothesis testing and experimental design for two-photon imaging data.PLoS Comput Biol. 2019 Oct 22;15(10):e1007473. doi: 10.1371/journal.pcbi.1007473. eCollection 2019 Oct. PLoS Comput Biol. 2019. PMID: 31639125 Free PMC article.
Abstract
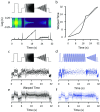
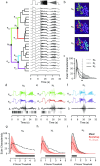
Variability, stochastic or otherwise, is a central feature of neural activity. Yet the means by which estimates of variation and uncertainty are derived from noisy observations of neural activity is often heuristic, with more weight given to numerical convenience than statistical rigour. For two-photon imaging data, composed of fundamentally probabilistic streams of photon detections, the problem is particularly acute. Here, we present a statistical pipeline for the inference and analysis of neural activity using Gaussian Process regression, applied to two-photon recordings of light-driven activity in ex vivo mouse retina. We demonstrate the flexibility and extensibility of these models, considering cases with non-stationary statistics, driven by complex parametric stimuli, in signal discrimination, hierarchical clustering and other inference tasks. Sparse approximation methods allow these models to be fitted rapidly, permitting them to actively guide the design of light stimulation in the midst of ongoing two-photon experiments.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248(4951):73–76. - PubMed
-
- Rasmussen CE, Williams CKI. Gaussian processes for machine learning. Cambridge, MA: MIT Press; 2006.
-
- Titsias M. Variational Learning of Inducing Variables in Sparse Gaussian Processes. Proceedings of the International Conference on Artificial Intelligence and Statistics (AISTATS). 2009;5:567–74.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

