Keratin 6, 16 and 17-Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis
- PMID: 31374826
- PMCID: PMC6721482
- DOI: 10.3390/cells8080807
Keratin 6, 16 and 17-Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis
Abstract
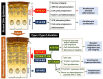
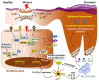
Located at the skin surface, keratinocytes (KCs) are constantly exposed to external stimuli and are the first responders to invading pathogens and injury. Upon skin injury, activated KCs secrete an array of alarmin molecules, providing a rapid and specific innate immune response against danger signals. However, dysregulation of the innate immune response of KCs may lead to uncontrolled inflammation and psoriasis pathogenesis. Keratins (KRT) are the major structural intermediate filament proteins in KCs and are expressed in a highly specific pattern at different differentiation stages of KCs. While KRT14-KRT5 is restricted to basal proliferative KCs, and KRT10-KRT1 is restricted to suprabasal differentiated KCs in normal skin epidermis, the wound proximal KCs downregulate KRT10-K1 and upregulate KRT16/KRT17-KRT6 upon skin injury. Recent studies have recognized KRT6/16/17 as key early barrier alarmins and upregulation of these keratins alters proliferation, cell adhesion, migration and inflammatory features of KCs, contributing to hyperproliferation and innate immune activation of KCs in response to an epidermal barrier breach, followed by the autoimmune activation of T cells that drives psoriasis. Here, we have reviewed how keratins are dysregulated during skin injury, their roles in wound repairs and in initiating the innate immune system and the subsequent autoimmune amplification that arises in psoriasis.
Keywords: autoimmune; barrier alarmins; epidermal keratinocytes; innate immune responses; keratins; proliferation; psoriasis; skin wounds.
Conflict of interest statement
The authors have no conflicts of interest.
Figures
Similar articles
-
Keratin 16 regulates innate immunity in response to epidermal barrier breach.Proc Natl Acad Sci U S A. 2013 Nov 26;110(48):19537-42. doi: 10.1073/pnas.1309576110. Epub 2013 Nov 11. Proc Natl Acad Sci U S A. 2013. PMID: 24218583 Free PMC article.
-
Ozone therapy promotes the differentiation of basal keratinocytes via increasing Tp63-mediated transcription of KRT10 to improve psoriasis.J Cell Mol Med. 2020 Apr;24(8):4819-4829. doi: 10.1111/jcmm.15160. Epub 2020 Mar 13. J Cell Mol Med. 2020. PMID: 32168425 Free PMC article.
-
Nrf2 Promotes Keratinocyte Proliferation in Psoriasis through Up-Regulation of Keratin 6, Keratin 16, and Keratin 17.J Invest Dermatol. 2017 Oct;137(10):2168-2176. doi: 10.1016/j.jid.2017.05.015. Epub 2017 May 30. J Invest Dermatol. 2017. PMID: 28576737
-
Type1 Interferons Potential Initiating Factors Linking Skin Wounds With Psoriasis Pathogenesis.Front Immunol. 2019 Jun 25;10:1440. doi: 10.3389/fimmu.2019.01440. eCollection 2019. Front Immunol. 2019. PMID: 31293591 Free PMC article. Review.
-
[Research advances on immunological properties and gene regulation of skin keratinocytes].Zhonghua Shao Shang Za Zhi. 2021 Jan 20;37(1):89-92. doi: 10.3760/cma.j.cn501120-20200106-00007. Zhonghua Shao Shang Za Zhi. 2021. PMID: 33499576 Review. Chinese.
Cited by
-
Keratins 6, 16, and 17 in Health and Disease: A Summary of Recent Findings.Curr Issues Mol Biol. 2024 Aug 6;46(8):8627-8641. doi: 10.3390/cimb46080508. Curr Issues Mol Biol. 2024. PMID: 39194725 Free PMC article. Review.
-
Histological and Molecular Biological Changes in Canine Skin Following Acute Radiation Therapy-Induced Skin Injury.Animals (Basel). 2024 Aug 29;14(17):2505. doi: 10.3390/ani14172505. Animals (Basel). 2024. PMID: 39272290 Free PMC article.
-
Modulation of Cellular Response to Different Parameters of the Rotating Magnetic Field (RMF)-An In Vitro Wound Healing Study.Int J Mol Sci. 2021 May 28;22(11):5785. doi: 10.3390/ijms22115785. Int J Mol Sci. 2021. PMID: 34071384 Free PMC article.
-
Application of quantitative proteomics to discover biomarkers for tick resistance in cattle.Front Immunol. 2023 Jan 30;14:1091066. doi: 10.3389/fimmu.2023.1091066. eCollection 2023. Front Immunol. 2023. PMID: 36793724 Free PMC article.
-
Recent Update on Immunopathogenesis of Psoriasis.Indian J Dermatol. 2022 Jul-Aug;67(4):360-373. doi: 10.4103/ijd.ijd_569_22. Indian J Dermatol. 2022. PMID: 36578729 Free PMC article.
References
-
- Driskell R.R., Lichtenberger B.M., Hoste E., Kretzschmar K., Simons B.D., Charalambous M., Ferron S.R., Herault Y., Pavlovic G., Ferguson-Smith A.C., et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277. doi: 10.1038/nature12783. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

