Physicochemical, Pharmacokinetic, and Toxicity Evaluation of Methoxy Poly(ethylene glycol)- b-Poly(d,l-Lactide) Polymeric Micelles Encapsulating Alpinumisoflavone Extracted from Unripe Cudrania tricuspidata Fruit
- PMID: 31374844
- PMCID: PMC6722910
- DOI: 10.3390/pharmaceutics11080366
Physicochemical, Pharmacokinetic, and Toxicity Evaluation of Methoxy Poly(ethylene glycol)- b-Poly(d,l-Lactide) Polymeric Micelles Encapsulating Alpinumisoflavone Extracted from Unripe Cudrania tricuspidata Fruit
Abstract
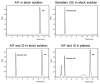
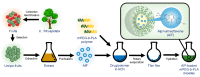
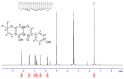
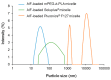
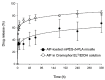
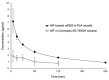
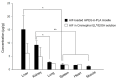
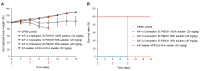
Alpinumisoflavone, a major compound in unripe Cudrania tricuspidata fruit is reported to exhibit numerous beneficial pharmacological activities, such as osteoprotective, antibacterial, estrogenic, anti-metastatic, atheroprotective, antioxidant, and anticancer effects. Despite its medicinal value, alpinumisoflavone is poorly soluble in water, which makes it difficult to formulate and administer intravenously (i.v.). To overcome these limitations, we used methoxy poly(ethylene glycol)-b-poly(d,l-lactide) (mPEG-b-PLA) polymeric micelles to solubilize alpinumisoflavone and increase its bioavailability, and evaluated their toxicity in vivo. Alpinumisoflavone-loaded polymeric micelles were prepared using thin-film hydration method, and their physicochemical properties were characterized for drug release, particle size, drug-loading (DL, %), and encapsulation efficiency (EE, %). The in vitro drug release profile was determined and the release rate of alpinumisoflavone from mPEG-b-PLA micelles was slower than that from drug solution, and sustained. Pharmacokinetic studies showed decreased total clearance and volume of distribution of alpinumisoflavone, whereas area under the curve (AUC) and bioavailability were significantly increased by incorporation in mPEG-b-PLA micelles. In vivo toxicity assay revealed that alpinumisoflavone-loaded mPEG-b-PLA micelles had no severe toxicity. In conclusion, we prepared an intravenous (i.v.) injectable alpinumisoflavone formulation, which was solubilized using mPEG-b-PLA micelles, and determined their physicochemical properties, pharmacokinetics, and toxicity profiles.
Keywords: alpinumisoflavone; mPEG-b-PLA micelle; pharmacokinetics; solubilization; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Xin L.T., Yue S.J., Fan Y.C., Wu J.S., Yan D., Guan H.S., Wang C.Y. Cudrania tricuspidata: An updated review on ethnomedicine, phytochemistry and pharmacology. RSC Adv. 2017;7:31807–31832. doi: 10.1039/C7RA04322H. - DOI
-
- Namkoong S., Kim T.J., Jang I.S., Kang K.W., Oh W.K., Park J. Alpinumisoflavone Induces Apoptosis and Suppresses Extracellular Signal-Regulated Kinases/Mitogen Activated Protein Kinase and Nuclear Factor-Kappa; B Pathways in Lung Tumor Cells. Biol. Pharm. Bull. 2011;34:203–208. doi: 10.1248/bpb.34.203. - DOI - PubMed
-
- Kingsford-Adaboh R., Dittrich B., Hubschle C.B., Gbewonyo W.S., Okamoto H., Kimura M., Ishida H. Invariom structure refinement, electrostatic potential and toxicity of 4-O-methylalpinumisoflavone, O,O-dimethylalpinumisoflavone and 5-O-methyl-4-O-(3-methylbut-2-en-1-yl)alpinumisoflavone. Acta Crystallogr. Sect. B Struct. Sci. 2006;62:843–849. doi: 10.1107/S0108768106019616. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

