Antibacterial Activities of Aliphatic Polyester Nanocomposites with Silver Nanoparticles and/or Graphene Oxide Sheets
- PMID: 31374855
- PMCID: PMC6724040
- DOI: 10.3390/nano9081102
Antibacterial Activities of Aliphatic Polyester Nanocomposites with Silver Nanoparticles and/or Graphene Oxide Sheets
Abstract
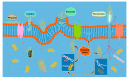
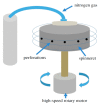
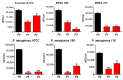
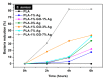
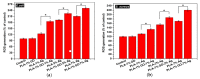
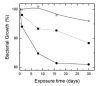
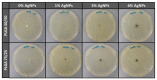
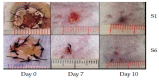
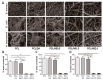
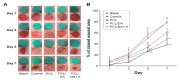
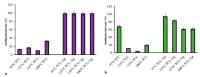
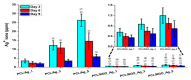
Aliphatic polyesters such as poly(lactic acid) (PLA), polycaprolactone (PCL) and poly(lactic-co-glycolic) acid (PLGA) copolymers have been widely used as biomaterials for tissue engineering applications including: bone fixation devices, bone scaffolds, and wound dressings in orthopedics. However, biodegradable aliphatic polyesters are prone to bacterial infections due to the lack of antibacterial moieties in their macromolecular chains. In this respect, silver nanoparticles (AgNPs), graphene oxide (GO) sheets and AgNPs-GO hybrids can be used as reinforcing nanofillers for aliphatic polyesters in forming antimicrobial nanocomposites. However, polymeric matrix materials immobilize nanofillers to a large extent so that they cannot penetrate bacterial membrane into cytoplasm as in the case of colloidal nanoparticles or nanosheets. Accordingly, loaded GO sheets of aliphatic polyester nanocomposites have lost their antibacterial functions such as nanoknife cutting, blanket wrapping and membrane phospholipid extraction. In contrast, AgNPs fillers of polyester nanocomposites can release silver ions for destroying bacterial cells. Thus, AgNPs fillers are more effective than loaded GO sheets of polyester nanocomposiites in inhibiting bacterial infections. Aliphatic polyester nanocomposites with AgNPs and AgNPs-GO fillers are effective to kill multi-drug resistant bacteria that cause medical device-related infections.
Keywords: Escherichia coli; aliphatic polyester; antibacterial activity; cellular viability; electrospinning; graphene oxide; osteoblast; scaffold; silver nanoparticle; staphylococcus aureus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





















References
-
- Sánchez-Salcedo S., Colilla M., Izquierdo-Barba I., Vallet-Regi M. Preventing bacterial adhesion on scaffolds for bone tissue engineering. Int. J. Bioprint. 2016;2:20–34. doi: 10.18063/IJB.2016.01.008. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

