Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650
- PMID: 31374872
- PMCID: PMC6723747
- DOI: 10.3390/pharmaceutics11080367
Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650
Abstract
Background: The epithelial layer of the nasal mucosa is the first barrier for drug permeation during intranasal drug delivery. With increasing interest for intranasal pathways, adequate in vitro models are required. Here, porcine olfactory (OEPC) and respiratory (REPC) primary cells were characterised against the nasal tumour cell line RPMI 2650.
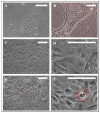
Methods: Culture conditions for primary cells from porcine nasal mucosa were optimized and the cells characterised via light microscope, RT-PCR and immunofluorescence. Epithelial barrier function was analysed via transepithelial electrical resistance (TEER), and FITC-dextran was used as model substance for transepithelial permeation. Beating cilia necessary for mucociliary clearance were studied by immunoreactivity against acetylated tubulin.
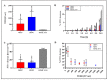
Results: OEPC and REPC barrier models differ in TEER, transepithelial permeation and MUC5AC levels. In contrast, RPMI 2650 displayed lower levels of MUC5AC, cilia markers and TEER, and higher FITC-dextran flux rates.
Conclusion: To screen pharmaceutical formulations for intranasal delivery in vitro, translational mucosal models are needed. Here, a novel and comprehensive characterisation of OEPC and REPC against RPMI 2650 is presented. The established primary models display an appropriate model for nasal mucosa with secreted MUC5AC, beating cilia and a functional epithelial barrier, which is suitable for long-term evaluation of sustained release dosage forms.
Keywords: RPMI 2650; barrier model; nose-to-brain; olfactory epithelium; primary cells; respiratory epithelium.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

