Hydrodynamics of Intravitreal Injections into Liquid Vitreous Substitutes
- PMID: 31374925
- PMCID: PMC6723562
- DOI: 10.3390/pharmaceutics11080371
Hydrodynamics of Intravitreal Injections into Liquid Vitreous Substitutes
Abstract
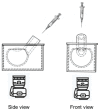
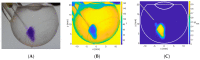
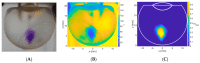
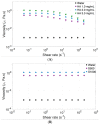
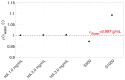
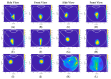
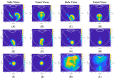
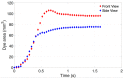
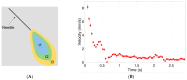
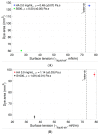
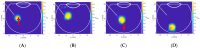
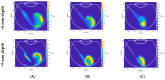
Intravitreal injections have become the cornerstone of retinal care and one of the most commonly performed procedures across all medical specialties. The impact of hydrodynamic forces of intravitreal solutions when injected into vitreous or vitreous substitutes has not been well described. While computational models do exist, they tend to underestimate the starting surface area of an injected bolus of a drug. Here, we report the dispersion profile of a dye bolus (50 µL) injected into different vitreous substitutes of varying viscosities, surface tensions, and volumetric densities. A novel 3D printed in vitro model of the vitreous cavity of the eye was designed to visualize the dispersion profile of solutions when injected into the following vitreous substitutes-balanced salt solution (BSS), sodium hyaluronate (HA), and silicone oils (SO)-using a 30G needle with a Reynolds number (Re) for injection ranging from approximately 189 to 677. Larger bolus surface areas were associated with faster injection speeds, lower viscosity of vitreous substitutes, and smaller difference in interfacial surface tensions. Boluses exhibited buoyancy when injected into standard S1000. The hydrodynamic properties of liquid vitreous substitutes influence the initial injected bolus dispersion profile and should be taken into account when simulating drug dispersion following intravitreal injection at a preclinical stage of development, to better inform formulations and performance.
Keywords: density; distribution; hyaluronic acid; hydrodynamics; intravitreal injection; surface tension; viscosity; vitreous.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Lau P.E., Jenkins K.S., Layton C.J. Current Evidence for the Prevention of Endophthalmitis in Anti-VEGF Intravitreal Injections. [(accessed on 24 July 2018)];J Ophthalmol. 2018 2018:1–8. doi: 10.1155/2018/8567912. Available online: https://www.hindawi.com/journals/joph/2018/8567912/ - DOI - PMC - PubMed
-
- Lamminsalo M., Taskinen E., Karvinen T., Subrizi A., Murtomäki L., Urtti A., Ranta V.P. Extended Pharmacokinetic Model of the Rabbit Eye for Intravitreal and Intracameral Injections of Macromolecules: Quantitative Analysis of Anterior and Posterior Elimination Pathways. [(accessed on 31 August 2018)];Pharm. Res. 2018 35:153. doi: 10.1007/s11095-018-2435-0. Available online: http://link.springer.com/10.1007/s11095-018-2435-0. - DOI - DOI - PubMed
-
- CATT Research Group. Martin D.F., Maguire M.G., Ying G., Grunwald J.E., Fine S.L., Jaffe G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. [(accessed on 19 May 2011)];N. Engl. J. Med. 2011 364:1897–1908. doi: 10.1016/j.ophtha.2012.03.053. Available online: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:new+englan.... - DOI - PMC - PubMed
-
- Tanzi M.G. Aflibercept: Bimonthly intravitreal injection for AMD. Pharm. Today. 2016;18:43. doi: 10.1016/j.ptdy.2016.11.019. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

