Soy Isoflavones Ameliorate Fatty Acid Metabolism of Visceral Adipose Tissue by Increasing the AMPK Activity in Male Rats with Diet-Induced Obesity (DIO)
- PMID: 31374939
- PMCID: PMC6696083
- DOI: 10.3390/molecules24152809
Soy Isoflavones Ameliorate Fatty Acid Metabolism of Visceral Adipose Tissue by Increasing the AMPK Activity in Male Rats with Diet-Induced Obesity (DIO)
Abstract
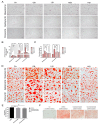
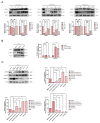
Soy isoflavones are natural active ingredients of soy plants that are beneficial to many metabolic diseases, especially obesity. Many studies have reported that obesity is closely related to visceral fatty acid metabolism, but the effect has not been well defined. In this study, we show that soy isoflavones improve visceral fatty acid metabolism in diet-induced obese male rats, which was indicated by reduced body weight and visceral fat cell area, as well as suppressed visceral fat synthesis and accelerated fat hydrolysis. We also found that common components of soy isoflavones, daidzein and genistein, were able to inhibit the lipid accumulation process in 3T3-L1 cells. Moreover, we showed that soy isoflavones can promote on AMP-activated protein kinase (AMPK) activity both in vivo and in vitro, which may be implicated in lipid metabolism regulation of soy isoflavones. Our study demonstrates the potential of soy isoflavones as a mechanism for regulating lipid homeostasis in visceral adipose tissue, proven to be beneficial for obesity treatment.
Keywords: AMPK; lipid homeostasis; soy isoflavones.
Conflict of interest statement
AMP-activated protein kinase
Figures


Similar articles
-
Doenjang, a fermented soybean paste, decreased visceral fat accumulation and adipocyte size in rats fed with high fat diet more effectively than nonfermented soybeans.J Med Food. 2012 Jan;15(1):1-9. doi: 10.1089/jmf.2010.1224. Epub 2011 Nov 14. J Med Food. 2012. PMID: 22082067
-
Soy Isoflavones Regulate Lipid Metabolism through an AKT/mTORC1 Pathway in Diet-Induced Obesity (DIO) Male Rats.Molecules. 2016 May 3;21(5):586. doi: 10.3390/molecules21050586. Molecules. 2016. PMID: 27153053 Free PMC article.
-
Improvement of Colonic Immune Function with Soy Isoflavones in High-Fat Diet-Induced Obese Rats.Molecules. 2019 Mar 22;24(6):1139. doi: 10.3390/molecules24061139. Molecules. 2019. PMID: 30909396 Free PMC article.
-
The effects of soy isoflavones on obesity.Exp Biol Med (Maywood). 2008 Sep;233(9):1066-80. doi: 10.3181/0712-MR-347. Epub 2008 Jun 5. Exp Biol Med (Maywood). 2008. PMID: 18535167 Review.
-
Soy Isoflavones and their Effects on Xenobiotic Metabolism.Curr Drug Metab. 2019;20(1):46-53. doi: 10.2174/1389200219666180427170213. Curr Drug Metab. 2019. PMID: 29708073 Review.
Cited by
-
The Role of Isoflavones in Type 2 Diabetes Prevention and Treatment-A Narrative Review.Int J Mol Sci. 2020 Dec 28;22(1):218. doi: 10.3390/ijms22010218. Int J Mol Sci. 2020. PMID: 33379327 Free PMC article. Review.
-
Ageratina adenophora Inhibits Spleen Immune Function in Rats via the Loss of the FRC Network and Th1-Th2 Cell Ratio Elevation.Toxins (Basel). 2021 Apr 26;13(5):309. doi: 10.3390/toxins13050309. Toxins (Basel). 2021. PMID: 33926136 Free PMC article.
-
Effects of Isoflavone Intake on Energy Requirement, Satiety, and Body Composition of Neutered Adult Cats.Animals (Basel). 2024 Dec 11;14(24):3574. doi: 10.3390/ani14243574. Animals (Basel). 2024. PMID: 39765478 Free PMC article.
-
Exercise and/or Genistein Treatment Impact Gut Microbiota and Inflammation after 12 Weeks on a High-Fat, High-Sugar Diet in C57BL/6 Mice.Nutrients. 2020 Nov 6;12(11):3410. doi: 10.3390/nu12113410. Nutrients. 2020. PMID: 33172007 Free PMC article.
-
Metabolic Impact of Flavonoids Consumption in Obesity: From Central to Peripheral.Nutrients. 2020 Aug 10;12(8):2393. doi: 10.3390/nu12082393. Nutrients. 2020. PMID: 32785059 Free PMC article. Review.
References
-
- Organization W.H. Global Strategy on Diet, Physical Activity and Health: Obesity and Overweight Fact sheet. Nutr. Newsl. 2005;48:292–302.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

