Design of Engineered Cyclodextrin Derivatives for Spontaneous Coating of Highly Porous Metal-Organic Framework Nanoparticles in Aqueous Media
- PMID: 31374940
- PMCID: PMC6723150
- DOI: 10.3390/nano9081103
Design of Engineered Cyclodextrin Derivatives for Spontaneous Coating of Highly Porous Metal-Organic Framework Nanoparticles in Aqueous Media
Abstract
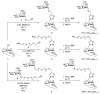
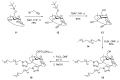
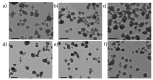
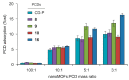
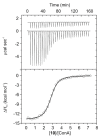
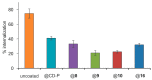
Nanosized metal-organic frameworks (nanoMOFs) MIL-100(Fe) are highly porous and biodegradable materials that have emerged as promising drug nanocarriers. A challenging issue concerns their surface functionalization in order to evade the immune system and to provide molecular recognition ability, so that they can be used for specific targeting. A convenient method for their coating with tetraethylene glycol, polyethylene glycol, and mannose residues is reported herein. The method consists of the organic solvent-free self-assembly on the nanoMOFs of building blocks based on β-cyclodextrin facially derivatized with the referred functional moieties, and multiple phosphate groups to anchor to the nanoparticles' surface. The coating of nanoMOFs with cyclodextrin phosphate without further functional groups led to a significant decrease of macrophage uptake, slightly improved by polyethylene glycol or mannose-containing cyclodextrin phosphate coating. More notably, nanoMOFs modified with tetraethylene glycol-containing cyclodextrin phosphate displayed the most efficient "stealth" effect. Mannose-coated nanoMOFs displayed a remarkably enhanced binding affinity towards a specific mannose receptor, such as Concanavalin A, due to the multivalent display of the monosaccharide, as well as reduced macrophage internalization. Coating with tetraethylente glycol of nanoMOFs after loading with doxorubicin is also described. Therefore, phosphorylated cyclodextrins offer a versatile platform to coat nanoMOFs in an organic solvent-free, one step manner, providing them with new biorecognition and/or "stealth" properties.
Keywords: MIL-100(Fe); isothermal titration calorimetry; macrophage; mannose; metal-organic frameworks; molecular recognition; multivalent effect; β-cyclodextrin; “stealth” effect.
Conflict of interest statement
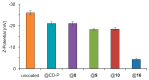
zeta potential
Figures

Similar articles
-
A non-covalent "click chemistry" strategy to efficiently coat highly porous MOF nanoparticles with a stable polymeric shell.Biochim Biophys Acta Gen Subj. 2017 Jun;1861(6):1606-1616. doi: 10.1016/j.bbagen.2017.01.016. Epub 2017 Jan 28. Biochim Biophys Acta Gen Subj. 2017. PMID: 28137620
-
Porous nanoparticles with engineered shells release their drug cargo in cancer cells.Int J Pharm. 2021 Dec 15;610:121230. doi: 10.1016/j.ijpharm.2021.121230. Epub 2021 Oct 28. Int J Pharm. 2021. PMID: 34718091
-
Comb-like dextran copolymers: A versatile strategy to coat highly porous MOF nanoparticles with a PEG shell.Carbohydr Polym. 2019 Nov 1;223:115085. doi: 10.1016/j.carbpol.2019.115085. Epub 2019 Jul 15. Carbohydr Polym. 2019. PMID: 31426973
-
Cyclodextrin-Modified inorganic materials for the construction of nanocarriers.Int J Pharm. 2017 Oct 15;531(2):621-639. doi: 10.1016/j.ijpharm.2017.06.080. Epub 2017 Jul 6. Int J Pharm. 2017. PMID: 28689967 Review.
-
Self-Assembly of Cyclodextrin-Coated Nanoparticles:Fabrication of Functional Nanostructures for Sensing and Delivery.Molecules. 2023 Jan 20;28(3):1076. doi: 10.3390/molecules28031076. Molecules. 2023. PMID: 36770743 Free PMC article. Review.
Cited by
-
One-Step Encapsulation of ortho-Disulfides in Functionalized Zinc MOF. Enabling Metal-Organic Frameworks in Agriculture.ACS Appl Mater Interfaces. 2021 Feb 24;13(7):7997-8005. doi: 10.1021/acsami.0c21488. Epub 2021 Feb 12. ACS Appl Mater Interfaces. 2021. PMID: 33577306 Free PMC article.
-
A comprehensive investigation of the interactions of human serum albumin with polymeric and hybrid nanoparticles.Drug Deliv Transl Res. 2024 Aug;14(8):2188-2202. doi: 10.1007/s13346-024-01578-x. Epub 2024 Apr 5. Drug Deliv Transl Res. 2024. PMID: 38578378
-
Cyclodextrin-Based Functional Glyconanomaterials.Nanomaterials (Basel). 2020 Dec 15;10(12):2517. doi: 10.3390/nano10122517. Nanomaterials (Basel). 2020. PMID: 33333914 Free PMC article. Review.
-
Acetic Acid-Modulated Room Temperature Synthesis of MIL-100 (Fe) Nanoparticles for Drug Delivery Applications.Int J Mol Sci. 2023 Jan 16;24(2):1757. doi: 10.3390/ijms24021757. Int J Mol Sci. 2023. PMID: 36675274 Free PMC article.
-
Doxorubicin-Loaded Metal-Organic Frameworks Nanoparticles with Engineered Cyclodextrin Coatings: Insights on Drug Location by Solid State NMR Spectroscopy.Nanomaterials (Basel). 2021 Apr 8;11(4):945. doi: 10.3390/nano11040945. Nanomaterials (Basel). 2021. PMID: 33917756 Free PMC article.
References
-
- Hoskins B.F., Robson R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989;111:5962–5964. doi: 10.1021/ja00197a079. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

