Highly Glycolytic Immortalized Human Dermal Microvascular Endothelial Cells are Able to Grow in Glucose-Starved Conditions
- PMID: 31374952
- PMCID: PMC6723428
- DOI: 10.3390/biom9080332
Highly Glycolytic Immortalized Human Dermal Microvascular Endothelial Cells are Able to Grow in Glucose-Starved Conditions
Abstract
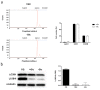
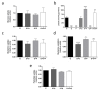
Endothelial cells form the inner lining of blood vessels, in a process known as angiogenesis. Excessive angiogenesis is a hallmark of several diseases, including cancer. The number of studies in endothelial cell metabolism has increased in recent years, and new metabolic targets for pharmacological treatment of pathological angiogenesis are being proposed. In this work, we wanted to address experimental evidence of substrate (namely glucose, glutamine and palmitate) dependence in immortalized dermal microvascular endothelial cells in comparison to primary endothelial cells. In addition, due to the lack of information about lactate metabolism in this specific type of endothelial cells, we also checked their capability of utilizing extracellular lactate. For fulfilling these aims, proliferation, migration, Seahorse, substrate uptake/utilization, and mRNA/protein expression experiments were performed. Our results show a high glycolytic capacity of immortalized dermal microvascular endothelial cells, but an early independence of glucose for cell growth, whereas a total dependence of glutamine to proliferate was found. Additionally, in contrast with reported data in other endothelial cell lines, these cells lack monocarboxylate transporter 1 for extracellular lactate incorporation. Therefore, our results point to the change of certain metabolic features depending on the endothelial cell line.
Keywords: MCT1; endothelial cells; glycolysis; lactate; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Monocarboxylate transporter 1 is a key player in glioma-endothelial cell crosstalk.Mol Carcinog. 2017 Dec;56(12):2630-2642. doi: 10.1002/mc.22707. Epub 2017 Aug 25. Mol Carcinog. 2017. PMID: 28762551
-
Lactate promotes glutamine uptake and metabolism in oxidative cancer cells.Cell Cycle. 2016;15(1):72-83. doi: 10.1080/15384101.2015.1120930. Cell Cycle. 2016. PMID: 26636483 Free PMC article.
-
Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis.PLoS One. 2012;7(3):e33418. doi: 10.1371/journal.pone.0033418. Epub 2012 Mar 13. PLoS One. 2012. PMID: 22428047 Free PMC article.
-
Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force.J Intern Med. 2013 Feb;273(2):156-65. doi: 10.1111/joim.12016. J Intern Med. 2013. PMID: 23216817 Review.
-
Metabolic coupling and the Reverse Warburg Effect in cancer: Implications for novel biomarker and anticancer agent development.Semin Oncol. 2017 Jun;44(3):198-203. doi: 10.1053/j.seminoncol.2017.10.004. Epub 2017 Oct 10. Semin Oncol. 2017. PMID: 29248131 Free PMC article. Review.
Cited by
-
Arterial Blood Pressure Features of Hypertensive Patients with Typical and Atypical 460 nm Skin Fluorescence Response to Transient Ischaemia.J Clin Med. 2023 Sep 10;12(18):5886. doi: 10.3390/jcm12185886. J Clin Med. 2023. PMID: 37762826 Free PMC article.
-
Glutamate dehydrogenase 1 mediated glutaminolysis sustains HCC cells survival under glucose deprivation.J Cancer. 2022 Jan 4;13(3):1061-1072. doi: 10.7150/jca.64195. eCollection 2022. J Cancer. 2022. PMID: 35154470 Free PMC article.
-
Glucose Favors Lipid Anabolic Metabolism in the Invasive Breast Cancer Cell Line MDA-MB-231.Biology (Basel). 2020 Jan 10;9(1):16. doi: 10.3390/biology9010016. Biology (Basel). 2020. PMID: 31936882 Free PMC article.
-
New insights in the targets of action of dimethyl fumarate in endothelial cells: effects on energetic metabolism and serine synthesis in vitro and in vivo.Commun Biol. 2023 Oct 25;6(1):1084. doi: 10.1038/s42003-023-05443-4. Commun Biol. 2023. PMID: 37880317 Free PMC article.
-
Aerobic glycolysis of vascular endothelial cells: a novel perspective in cancer therapy.Mol Biol Rep. 2024 Jun 1;51(1):717. doi: 10.1007/s11033-024-09588-1. Mol Biol Rep. 2024. PMID: 38824197 Free PMC article. Review.
References
-
- Folkman J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. - PubMed

