APEX1 Expression as a Potential Diagnostic Biomarker of Clear Cell Renal Cell Carcinoma and Hepatobiliary Carcinomas
- PMID: 31375000
- PMCID: PMC6723795
- DOI: 10.3390/jcm8081151
APEX1 Expression as a Potential Diagnostic Biomarker of Clear Cell Renal Cell Carcinoma and Hepatobiliary Carcinomas
Abstract
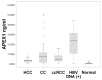
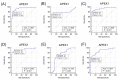
Apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APEX1) has been known to play key roles in DNA repair, the regulation of diverse transcriptional activity, and cellular responses to redox activity. This study aimed to examine serum APEX1 (s-APEX1) expression as a possible screening biomarker for clear cell renal cell carcinoma (ccRCC), hepatocellular carcinoma (HCC), and proximal and distal cholangiocarcinoma (CC). A total of 216 frozen serum samples were collected from 39 healthy control cases, 32 patients with ≥58 copies/mL of hepatitis B viral DNA (HBV DNA (+)), 40 ccRCC cases, 59 HCC cases, and 46 CC cases. The serum samples were examined for s-APEX1 concentration by enzyme-linked immunosorbent assay. The association of APEX1 expression with clinicopathological characteristics was also studied by immunohistochemical staining in 106 ccRCC, 131 HCC, and 32 intrahepatic CC cases. The median s-APEX1 concentrations of the HCC, CC, ccRCC, healthy control, and HBV DNA (+) groups were 0.294, 0.710, 0.474, 0.038, and 2.384 ng/mL, respectively (p < 0.001). Univariate and multivariate analyses revealed that increased cytoplasmic APEX1 expression led to a shorter disease-free survival period in HCC and CC cases. We suggest that the s-APEX1 level could be a potential diagnostic biomarker of ccRCC, HCC, and CC. Additionally, cytoplasmic APEX1 expression in cancer cells could be used to predict relapses in patients with HCC or CC.
Keywords: apurinic/apyrimidinic endonuclease 1/redox effector factor 1; cholangiocarcinoma; clear cell renal cell carcinoma; diagnosis; hepatocellular carcinoma.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; the collection, analyses, or interpretation of data; the writing of the manuscript, or in the decision to publish these results.
Figures


References
-
- O’Hara A.M., Bhattacharyya A., Mifflin R.C., Smith M.F., Ryan K.A., Scott K.G., Naganuma M., Casola A., Izumi T., Mitra S., et al. Interleukin-8 induction by Helicobacter pylori in gastric epithelial cells is dependent on apurinic/apyrimidinic endonuclease-1/redox factor-1. J. Immunol. 2006;177:7990–7999. doi: 10.4049/jimmunol.177.11.7990. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

