Independent Evolution with the Gene Flux Originating from Multiple Xanthomonas Species Explains Genomic Heterogeneity in Xanthomonas perforans
- PMID: 31375496
- PMCID: PMC6805091
- DOI: 10.1128/AEM.00885-19
Independent Evolution with the Gene Flux Originating from Multiple Xanthomonas Species Explains Genomic Heterogeneity in Xanthomonas perforans
Abstract
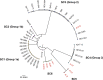
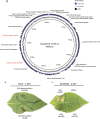
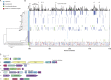
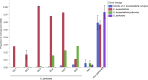
Xanthomonas perforans is the predominant pathogen responsible for bacterial leaf spot of tomato and X. euvesicatoria for that of pepper in the southeast United States. Previous studies have indicated significant changes in the X. perforans population collected from Florida tomato fields over the span of 2 decades, including a shift in race and diversification into three phylogenetic groups driven by genome-wide homologous-recombination events derived from X. euvesicatoria In our sampling of Xanthomonas strains associated with bacterial spot disease in Alabama, we were readily able to isolate X. perforans from symptomatic pepper plants grown in several Alabama counties, indicating a recent shift in the host range of the pathogen. To investigate the diversity of these pepper-pathogenic strains and their relation to populations associated with tomatoes grown in the southeast United States, we sequenced the genomes of eight X. perforans strains isolated from tomatoes and peppers grown in Alabama and compared them with previously published genome data available from GenBank. Surprisingly, reconstruction of the X. perforans core genome revealed the presence of two novel genetic groups in Alabama that each harbored a different transcription activation-like effector (TALE). While one TALE, AvrHah1, was associated with an emergent lineage pathogenic to both tomato and pepper, the other was identified as a new class within the AvrBs3 family, here designated PthXp1, and was associated with enhanced symptom development on tomato. Examination of patterns of homologous recombination across the larger X. euvesicatoria species complex revealed a dynamic pattern of gene flow, with multiple donors of Xanthomonas spp. associated with diverse hosts of isolation.IMPORTANCE Bacterial leaf spot of tomato and pepper is an endemic plant disease with a global distribution. In this study, we investigated the evolutionary processes leading to the emergence of novel X. perforans lineages identified in Alabama. While one lineage was isolated from symptomatic tomato and pepper plants, confirming the host range expansion of X. perforans, the other lineage was isolated from tomato and acquired a novel transcription activation-like effector, here designated PthXp1. Functional analysis of PthXp1 indicated that it does not induce Bs4-mediated resistance in tomato and contributes to virulence, providing an adaptive advantage to strains on tomato. Our findings also show that different phylogenetic groups of the pathogen have experienced independent recombination events originating from multiple Xanthomonas species. This suggests a continuous gene flux between related xanthomonads associated with diverse plant hosts that results in the emergence of novel pathogen lineages and associated phenotypes, including host range.
Keywords: TAL effector; Xanthomonas; emerging; host specificity; novel lineages; recombination.
Copyright © 2019 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

