Zika Virus Infection Induces DNA Damage Response in Human Neural Progenitors That Enhances Viral Replication
- PMID: 31375586
- PMCID: PMC6798117
- DOI: 10.1128/JVI.00638-19
Zika Virus Infection Induces DNA Damage Response in Human Neural Progenitors That Enhances Viral Replication
Abstract
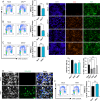
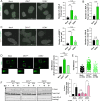
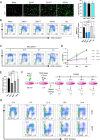
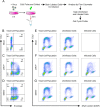
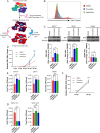
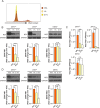
Zika virus (ZIKV) infection attenuates the growth of human neural progenitor cells (hNPCs). As these hNPCs generate the cortical neurons during early brain development, the ZIKV-mediated growth retardation potentially contributes to the neurodevelopmental defects of the congenital Zika syndrome. Here, we investigate the mechanism by which ZIKV manipulates the cell cycle in hNPCs and the functional consequence of cell cycle perturbation on the replication of ZIKV and related flaviviruses. We demonstrate that ZIKV, but not dengue virus (DENV), induces DNA double-strand breaks (DSBs), triggering the DNA damage response through the ATM/Chk2 signaling pathway while suppressing the ATR/Chk1 signaling pathway. Furthermore, ZIKV infection impedes the progression of cells through S phase, thereby preventing the completion of host DNA replication. Recapitulation of the S-phase arrest state with inhibitors led to an increase in ZIKV replication, but not of West Nile virus or DENV. Our data identify ZIKV's ability to induce DSBs and suppress host DNA replication, which results in a cellular environment favorable for its replication.IMPORTANCE Clinically, Zika virus (ZIKV) infection can lead to developmental defects in the cortex of the fetal brain. How ZIKV triggers this event in developing neural cells is not well understood at a molecular level and likely requires many contributing factors. ZIKV efficiently infects human neural progenitor cells (hNPCs) and leads to growth arrest of these cells, which are critical for brain development. Here, we demonstrate that infection with ZIKV, but not dengue virus, disrupts the cell cycle of hNPCs by halting DNA replication during S phase and inducing DNA damage. We further show that ZIKV infection activates the ATM/Chk2 checkpoint but prevents the activation of another checkpoint, the ATR/Chk1 pathway. These results unravel an intriguing mechanism by which an RNA virus interrupts host DNA replication. Finally, by mimicking virus-induced S-phase arrest, we show that ZIKV manipulates the cell cycle to benefit viral replication.
Keywords: DNA damage; DNA damage checkpoints; DNA damage response; DNA replication; S phase; Zika virus; cell cycle; neural progenitors.
Copyright © 2019 American Society for Microbiology.
Figures


References
-
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho CY, Wen Z, Christian KM, Shi PY, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming GL. 2016. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165:1238–1254. doi: 10.1016/j.cell.2016.04.032. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

