Measles Virus Forms Inclusion Bodies with Properties of Liquid Organelles
- PMID: 31375591
- PMCID: PMC6803276
- DOI: 10.1128/JVI.00948-19
Measles Virus Forms Inclusion Bodies with Properties of Liquid Organelles
Abstract
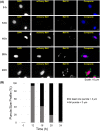
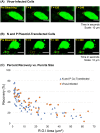
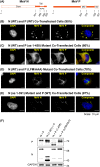
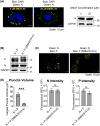
Nonsegmented negative-strand RNA viruses, including measles virus (MeV), a member of the Paramyxoviridae family, are assumed to replicate in cytoplasmic inclusion bodies. These cytoplasmic viral factories are not membrane bound, and they serve to concentrate the viral RNA replication machinery. Although inclusion bodies are a prominent feature in MeV-infected cells, their biogenesis and regulation are not well understood. Here, we show that infection with MeV triggers inclusion body formation via liquid-liquid phase separation (LLPS), a process underlying the formation of membraneless organelles. We find that the viral nucleoprotein (N) and phosphoprotein (P) are sufficient to trigger MeV phase separation, with the C-terminal domains of the viral N and P proteins playing a critical role in the phase transition. We provide evidence suggesting that the phosphorylation of P and dynein-mediated transport facilitate the growth of these organelles, implying that they may have key regulatory roles in the biophysical assembly process. In addition, our findings support the notion that these inclusions change from liquid to gel-like structures as a function of time after infection, leaving open the intriguing possibility that the dynamics of these organelles can be tuned during infection to optimally suit the changing needs during the viral replication cycle. Our study provides novel insight into the process of formation of viral inclusion factories, and taken together with earlier studies, suggests that Mononegavirales have broadly evolved to utilize LLPS as a common strategy to assemble cytoplasmic replication factories in infected cells.IMPORTANCE Measles virus remains a pathogen of significant global concern. Despite an effective vaccine, outbreaks continue to occur, and globally ∼100,000 measles-related deaths are seen annually. Understanding the molecular basis of virus-host interactions that impact the efficiency of virus replication is essential for the further development of prophylactic and therapeutic strategies. Measles virus replication occurs in the cytoplasm in association with discrete bodies, though little is known of the nature of the inclusion body structures. We recently established that the cellular protein WD repeat-containing protein 5 (WDR5) enhances MeV growth and is enriched in cytoplasmic viral inclusion bodies that include viral proteins responsible for RNA replication. Here, we show that MeV N and P proteins are sufficient to trigger the formation of WDR5-containing inclusion bodies, that these structures display properties characteristic of phase-separated liquid organelles, and that P phosphorylation together with the host dynein motor affect the efficiency of the liquid-liquid phase separation process.
Keywords: WD repeat-containing protein WDR5; inclusion body; innate immunity; liquid-liquid phase separation; measles virus.
Copyright © 2019 American Society for Microbiology.
Figures




References
-
- Lamb RA, Parks GD. 2013. Paramyxoviridae, p 957–995. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Griffin D. 2013. Measles, p 1042–1069. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Bourhis J-M, Johansson K, Receveur-Bréchot V, Oldfield CJ, Dunker KA, Canard B, Longhi S. 2004. The C-terminal domain of measles virus nucleoprotein belongs to the class of intrinsically disordered proteins that fold upon binding to their physiological partner. Virus Res 99:157–167. doi: 10.1016/j.virusres.2003.11.007. - DOI - PubMed

