Molecular mimicry between Anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk
- PMID: 31375628
- PMCID: PMC6708327
- DOI: 10.1073/pnas.1902623116
Molecular mimicry between Anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk
Abstract
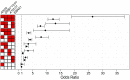
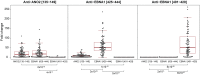
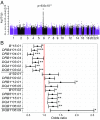
Multiple sclerosis (MS) is a chronic inflammatory, likely autoimmune disease of the central nervous system with a combination of genetic and environmental risk factors, among which Epstein-Barr virus (EBV) infection is a strong suspect. We have previously identified increased autoantibody levels toward the chloride-channel protein Anoctamin 2 (ANO2) in MS. Here, IgG antibody reactivity toward ANO2 and EBV nuclear antigen 1 (EBNA1) was measured using bead-based multiplex serology in plasma samples from 8,746 MS cases and 7,228 controls. We detected increased anti-ANO2 antibody levels in MS (P = 3.5 × 10-36) with 14.6% of cases and 7.8% of controls being ANO2 seropositive (odds ratio [OR] = 1.6; 95% confidence intervals [95%CI]: 1.5 to 1.8). The MS risk increase in ANO2-seropositive individuals was dramatic when also exposed to 3 known risk factors for MS: HLA-DRB1*15:01 carriage, absence of HLA-A*02:01, and high anti-EBNA1 antibody levels (OR = 24.9; 95%CI: 17.9 to 34.8). Reciprocal blocking experiments with ANO2 and EBNA1 peptides demonstrated antibody cross-reactivity, mapping to ANO2 [aa 140 to 149] and EBNA1 [aa 431 to 440]. HLA gene region was associated with anti-ANO2 antibody levels and HLA-DRB1*04:01 haplotype was negatively associated with ANO2 seropositivity (OR = 0.6; 95%CI: 0.5 to 0.7). Anti-ANO2 antibody levels were not increased in patients from 3 other inflammatory disease cohorts. The HLA influence and the fact that specific IgG production usually needs T cell help provides indirect evidence for a T cell ANO2 autoreactivity in MS. We propose a hypothesis where immune reactivity toward EBNA1 through molecular mimicry with ANO2 contributes to the etiopathogenesis of MS.
Keywords: ANO2; Anoctamin 2; Epstein-Barr virus; molecular mimicry; multiple sclerosis.
Conflict of interest statement
Conflict of interest statement: Outside this work, T.O. has received unrestricted MS research grants, lecture and/or advisory board honoraria from: Biogen, Novartis, Merck, Sanofi, and Roche. Outside this work, P.K. is working at Roche Diagnostics in unrelated projects.
Figures

References
-
- Olsson T., Piehl F., “The immunobiology of multiple sclerosis” in Encyclopedia of Immunobiology, Ratcliffe M. J. H., Ed. (Academic, Oxford, 2016), vol. 5, pp. 180–191.
-
- Olsson T., Barcellos L. F., Alfredsson L., Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 13, 25–36 (2017). - PubMed
-
- Kockum I., Alfredsson L., Olsson T., “Genetic and environmental risk factors for multiple sclerosis—A role for interaction analysis” in Between the Lines of Genetic Code, Genetic Interactions in Understanding Disease and Complex Phenotypes, Padyukov L., Ed. (Academic Press, 2014), pp. 101–114.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

