Long-term tolerance of islet allografts in nonhuman primates induced by apoptotic donor leukocytes
- PMID: 31375697
- PMCID: PMC6677762
- DOI: 10.1038/s41467-019-11338-y
Long-term tolerance of islet allografts in nonhuman primates induced by apoptotic donor leukocytes
Abstract
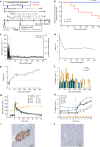
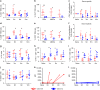
Immune tolerance to allografts has been pursued for decades as an important goal in transplantation. Administration of apoptotic donor splenocytes effectively induces antigen-specific tolerance to allografts in murine studies. Here we show that two peritransplant infusions of apoptotic donor leukocytes under short-term immunotherapy with antagonistic anti-CD40 antibody 2C10R4, rapamycin, soluble tumor necrosis factor receptor and anti-interleukin 6 receptor antibody induce long-term (≥1 year) tolerance to islet allografts in 5 of 5 nonsensitized, MHC class I-disparate, and one MHC class II DRB allele-matched rhesus macaques. Tolerance in our preclinical model is associated with a regulatory network, involving antigen-specific Tr1 cells exhibiting a distinct transcriptome and indirect specificity for matched MHC class II and mismatched class I peptides. Apoptotic donor leukocyte infusions warrant continued investigation as a cellular, nonchimeric and translatable method for inducing antigen-specific tolerance in transplantation.
Conflict of interest statement
S.R. and C.B. received research support for projects not reported in this article by Diabetes-Free, Inc., an organization that may gain or lose financially through this publication. M.L.G. is a paid consultant for Otsuka Pharmaceutical Factory, Inc. S.D.M. is a co-founder, member of the SAB and a paid consultant for Cour Pharmaceuticals Development Company; a member of the SAB and a paid consultant for NextCure Inc.; and a paid consultant for Kite Pharmaceuticals. S.D.M. and X.L. are inventors on issued patent no. US 8,734,786 B2 submitted by Northwestern University that covers the use of ECDI-fixed cell tolerance as a method for preventing allograft rejection. B.J.H. has an equity interest in and serves as an executive officer of Diabetes-Free, an organization that may commercially benefit from the results of this research. This interest has been reviewed and managed by the University of Minnesota in accordance with its Conflict of Interest policies. The other authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

