Short-term CFTR inhibition reduces islet area in C57BL/6 mice
- PMID: 31375720
- PMCID: PMC6677757
- DOI: 10.1038/s41598-019-47745-w
Short-term CFTR inhibition reduces islet area in C57BL/6 mice
Abstract
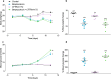
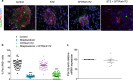
Cystic fibrosis-related diabetes (CFRD) worsens CF lung disease leading to early mortality. Loss of beta cell area, even without overt diabetes or pancreatitis is consistently observed. We investigated whether short-term CFTR inhibition was sufficient to impact islet morphology and function in otherwise healthy mice. CFTR was inhibited in C57BL/6 mice via 8-day intraperitoneal injection of CFTRinh172. Animals had a 7-day washout period before measures of hormone concentration or islet function were performed. Short-term CFTR inhibition increased blood glucose concentrations over the course of the study. However, glucose tolerance remained normal without insulin resistance. CFTR inhibition caused marked reductions in islet size and in beta cell and non-beta cell area within the islet, which resulted from loss of islet cell size rather than islet cell number. Significant reductions in plasma insulin concentrations and pancreatic insulin content were also observed in CFTR-inhibited animals. Temporary CFTR inhibition had little long-term impact on glucose-stimulated, or GLP-1 potentiated insulin secretion. CFTR inhibition has a rapid impact on islet area and insulin concentrations. However, islet cell number is maintained and insulin secretion is unaffected suggesting that early administration of therapies aimed at sustaining beta cell mass may be useful in slowing the onset of CFRD.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

