Pharmacokinetics in children with chronic kidney disease
- PMID: 31375913
- PMCID: PMC7248054
- DOI: 10.1007/s00467-019-04304-9
Pharmacokinetics in children with chronic kidney disease
Abstract
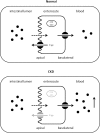
In children, the main causes of chronic kidney disease (CKD) are congenital diseases and glomerular disorders. CKD is associated with multiple physiological changes and may therefore influence various pharmacokinetic (PK) parameters. A well-known consequence of CKD on pharmacokinetics is a reduction in renal clearance due to a decrease in the glomerular filtration rate. The impact of renal impairment on pharmacokinetics is, however, not limited to a decreased elimination of drugs excreted by the kidney. In fact, renal dysfunction may lead to modifications in absorption, distribution, transport, and metabolism as well. Currently, insufficient evidence is available to guide dosing decisions on many commonly used drugs. Moreover, the impact of maturation on drug disposition and action should be taken into account when selecting and dosing drugs in the pediatric population. Clinicians should take PK changes into consideration when selecting and dosing drugs in pediatric CKD patients in order to avoid toxicity and increase efficiency of drugs in this population. The aim of this review is to summarize known PK changes in relation to CKD and to extrapolate available knowledge to the pediatric CKD population to provide guidance for clinical practice.
Keywords: Absorption; CKD; Children; Distribution; Excretion; Metabolism; Pharmacokinetics.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Ardissino G, Dacco V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, Marra G, Edefonti A, Sereni F, ItalKid Project Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. 2003;111:e382–e387. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

