Resveratrol Directly Controls the Activity of Neuronal Ryanodine Receptors at the Single-Channel Level
- PMID: 31376069
- PMCID: PMC6980486
- DOI: 10.1007/s12035-019-01705-7
Resveratrol Directly Controls the Activity of Neuronal Ryanodine Receptors at the Single-Channel Level
Abstract
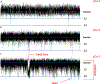
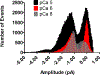
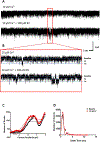
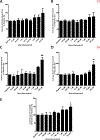
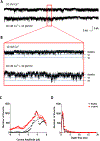
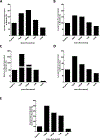
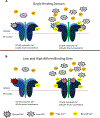
Calcium ion dyshomeostasis contributes to the progression of many neurodegenerative diseases and represents a target for the development of neuroprotective therapies, as reported by Duncan et al. (Molecules 15(3):1168-95, 2010), LaFerla (Nat Rev Neurosci 3(11):862-72, 2002), and Niittykoshi et al. (Invest Ophthalmol Vis Sci 51(12):6387-93, 2010). Dysfunctional ryanodine receptors contribute to calcium ion dyshomeostasis and potentially to the pathogenesis of neurodegenerative diseases by generating abnormal calcium ion release from the endoplasmic reticulum, according to Bruno et al. (Neurobiol Aging 33(5):1001 e1-6, 2012) and Stutzmann et al. (J Neurosci 24(2):508-13, 2004). Since ryanodine receptors share functional and structural similarities with potassium channels, as reported by Lanner et al. (Cold Spring Harb Perspect Biol 2(11):a003996, 2010), and small molecules with anti-oxidant properties, such as resveratrol (3,5,4'-trihydroxy-trans-stilbene), directly control the activity of potassium channels, according to Wang et al. (J Biomed Sci 23(1):47, 2016), McCalley et al. (Molecules 19(6):7327-40, 2014), Novakovic et al. (Mol Hum Reprod 21(6):545-51, 2015), Li et al. (Cardiovasc Res 45(4):1035-45, 2000), Gopalakrishnan et al. (Br J Pharmacol 129(7):1323-32, 2000), and Hambrock et al. (J Biol Chem 282(5):3347-56, 2007), we hypothesized that trans-resveratrol can modulate intracellular calcium signaling through direct binding and functional regulation of ryanodine receptors. The goal of our study was to identify and measure the control of ryanodine receptor activity by trans-resveratrol. Mechanisms of calcium signaling mediated by the direct interaction between trans-resveratrol and ryanodine receptors were identified and measured with single-channel electrophysiology. Addition of trans-resveratrol to the cytoplasmic face of the ryanodine receptor increased single-channel activity at physiological and elevated pathophysiological cytoplasmic calcium ion concentrations. The open probability of the channel increases after interacting with the small molecule in a dose-dependent manner, but remains also dependent on the concentration of its physiological ligand, cytoplasmic-free calcium ions. This study provides the first evidence of a direct functional interaction between trans-resveratrol and ryanodine receptors. Such functional control of ryanodine receptors by trans-resveratrol as a novel mechanism of action could provide additional rationales for the development of novel therapeutic strategies to treat and prevent neurodegenerative diseases.
Keywords: 3,5,4′-Trihydroxy-trans-stilbene; Ca2+; Calcium; Electrophysiology; Neurodegeneration; Neuroprotection; Neuroprotective therapies; Pharmacology; RyR; Trans-resveratrol.
Figures
Similar articles
-
A computational analysis of localized Ca2+-dynamics generated by heterogeneous release sites.Bull Math Biol. 2009 Oct;71(7):1543-79. doi: 10.1007/s11538-009-9413-y. Epub 2009 May 14. Bull Math Biol. 2009. PMID: 19440797
-
Control of Neuronal Ryanodine Receptor-Mediated Calcium Signaling by Calsenilin.Mol Neurobiol. 2019 Jan;56(1):525-534. doi: 10.1007/s12035-018-1080-2. Epub 2018 May 5. Mol Neurobiol. 2019. PMID: 29730765 Free PMC article.
-
Vesl/Homer proteins regulate ryanodine receptor type 2 function and intracellular calcium signaling.Cell Calcium. 2003 Sep;34(3):261-9. doi: 10.1016/s0143-4160(03)00112-x. Cell Calcium. 2003. PMID: 12887973
-
Neuronal Ryanodine Receptors in Development and Aging.Mol Neurobiol. 2018 Feb;55(2):1183-1192. doi: 10.1007/s12035-016-0375-4. Epub 2017 Jan 19. Mol Neurobiol. 2018. PMID: 28102470 Review.
-
Ryanodine Receptors: A Potential Treatment Target in Various Neurodegenerative Disease.Cell Mol Neurobiol. 2021 Nov;41(8):1613-1624. doi: 10.1007/s10571-020-00936-w. Epub 2020 Aug 24. Cell Mol Neurobiol. 2021. PMID: 32833122 Free PMC article. Review.
References
-
- LaFerla FM, Calcium Dyshomeostasis and Intracellular Signalling in Alzheimer’s Disease . Nat. Rev. Neurosci 2002, 3 (11), 862–72. - PubMed
-
- Niittykoski M; Kalesnykas G; Larsson KP; Kaarniranta K; Akerman KE; Uusitalo H, Altered Calcium Signaling in an Experimental Model of Glaucoma. Invest. Ophthalmol. Vis. Sci 2010, 51 (12), 6387–93. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

