Real-World Rivaroxaban and Apixaban Levels in Asian Patients With Atrial Fibrillation
- PMID: 31376150
- PMCID: PMC6977317
- DOI: 10.1002/cpt.1601
Real-World Rivaroxaban and Apixaban Levels in Asian Patients With Atrial Fibrillation
Abstract
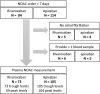
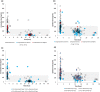
This study aims to measure the plasma levels of rivaroxaban and apixaban among Asian patients with atrial fibrillation and compare the results with expected drug levels from clinical studies. A total of 73 patients taking rivaroxaban and 105 patients taking apixaban were enrolled. Peak and trough levels were measured using ultra-high performance liquid chromatography with tandem mass spectrometry. The percentage of those with drug levels within the expected range reported in clinical studies was significantly higher in the apixaban group than in the rivaroxaban group, both for trough (84.8% vs. 64.4%; P = 0.002) and peak levels (76.9% vs. 33.8%; P < 0.001). After adjusting for age, sex, kidney function, appropriate dose, and adherence, patients taking rivaroxaban were still less likely to have peak and trough levels within the expected drug levels. Our real-world data suggests that Asian patients taking rivaroxaban are more likely to have out-of-expected drug levels than those taking apixaban.
© 2019 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
References
-
- January, C.T. et al 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 74, 104–132 (2019). - PubMed
-
- Kirchhof, P. et al 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 37, 2893–2962 (2016). - PubMed
-
- Steffel, J. et al The 2018 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 39, 1330–1393 (2018). - PubMed
-
- Tanigawa, T. et al Model‐based dose selection for phase III rivaroxaban study in Japanese patients with non‐valvular atrial fibrillation. Drug Metab. Pharmacokinet. 28, 59–70 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

