Suboptimal UVA attenuation by broad spectrum sunscreens under outdoor solar conditions contributes to lifetime UVA burden
- PMID: 31376300
- PMCID: PMC7853154
- DOI: 10.1111/phpp.12503
Suboptimal UVA attenuation by broad spectrum sunscreens under outdoor solar conditions contributes to lifetime UVA burden
Abstract
Background: Broad spectrum sunscreens with a sun protection factor (SPF) of 15 or greater are indicated to decrease the risk of skin cancer and early skin aging caused by the sun if used as directed with other sun protection measures. To determine whether sunscreen product performance is compromised under solar exposure and to test spectral uniformity of protection across the UVA spectrum, we tested broad spectrum sunscreens with a variety of active pharmaceutical ingredients (APIs) and in a variety of dosage forms.
Methods: A cross-sectional market survey of 32 sunscreen drug products containing either organic or inorganic APIs with SPFs of 15, 30, 50, and 70 was tested. UV doses were delivered via natural sun in Silver Spring, Maryland between June and September of 2017.
Results: Of the 32 sunscreen drug products, 6 products failed to meet their broad spectrum claim under solar exposure. Using FDA's new proposal to strengthen sunscreen broad spectrum requirements, spectral uniformity based on the mean sunscreen absorbance of UVA1(340-400 nm)/UV (290-400 nm) indicated that ~40% of sunscreen drug products tested had suboptimal UVA protection.
Conclusion: US consumers may unknowingly be receiving up to 36% more transmitted UVA when selecting between similarly labeled broad spectrum sunscreen drug products with equivalent SPF values. FDA's new proposal may help decrease consumers' overall lifetime UVA burden. Spectral absorbance data on sunscreen performance can be used to further improve the coupling of broad spectrum protection to a product's SPF value so that consumers have improved proportional increases in UV protection.
Keywords: broad spectrum; skin cancer; sun protection factor; sunscreen; ultraviolet radiation.
Published 2019. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Conflict of Interest:
Figures
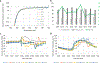

References
-
- Tadokoro T, Kobayashi N, Zmudzka BZ, et al. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J. 2003;17:1177–1179. - PubMed
-
- U.S. Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. Washington, DC: U.S. Dept of Health and Human Services, Office of the Surgeon General; 2014. - PubMed
-
- U.S. Food and Drug Administration. Sunscreen Drug Products for Over-The-Counter Human Use; Proposed Rule. In: Department of Health and Human Services, ed. Vol 84: Federal Register; 2019:6204–6275.
-
- 29th Hawaii State Legislature. Environment; Water Pollution; Sunscreen; Oxybenzone; Octinoxate; Sale; Distribution; Prohibition. SB2571 SD2 HD2 CD1 2018; 1–6. Available at: https://www.capitol.hawaii.gov/Archives/measure_indiv_Archives.aspx?bill.... Accessed June 6, 2019.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

