Characterization and In Silico Analysis of The Structural Features of G-CSF Derived from Lysates of Escherichia coli
- PMID: 31376324
- PMCID: PMC6722442
- DOI: 10.22074/cellj.2020.6158
Characterization and In Silico Analysis of The Structural Features of G-CSF Derived from Lysates of Escherichia coli
Abstract
Objective: Granulocyte colony-stimulating factor (G-CSF) has a wide variety of functions including stimulation of hematopoiesis and proliferation of granulocyte progenitor cells. Recombinant human G-CSF (rh-G-CSF) is used for treatment of neutropenia in patients receiving chemotherapy. The mature bloodstream neutrophils express G-CSF receptor (G-CSFR), presenting a significant and specific mechanism for circulating G-CSF clearance. Computational studies are essential bioinformatics methods used for characterization of proteins with regard to their physicochemical properties and 3D configuration, as well as protein-ligand interactions for recombinant drugs. We formerly produced rh-G-CSF in E. coli and showed that the isolated protein had unacceptable biological activity in mice. In the present paper, we aimed to characterize the purified rh-G-CSF by analytical tests and developed an in vivo model by computational modelling of G-CSF.
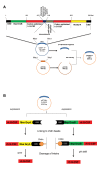
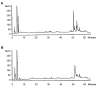
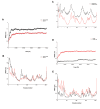
Materials and methods: In this experimental study, we analyzed the purified G-CSF using the analytical experiments. Then, the crystalline structure was extracted from Protein Data Bank (PDB) and molecular dynamics (MD) simulation was performed using Gromacs 5.1 package under an Amber force field. The importance of amino acid contents of G-CSF, to bind the respective receptor was also detected; moreover, the effect of dithiothreitol (DTT) used in G-CSF purification was studied.
Results: The results revealed that characteristics of the produced recombinant G-CSF were comparable with those of the standard G-CSF and the recombinant G-CSF with the residual amino acid was stable. Also, purification conditions (DTT and existence of extra cysteine) had a significant effect on the stability and functionality of the produced G-CSF.
Conclusion: Experimental and in silico analyses provided good information regarding the function and characteristics of our recombinant G-CSF which could be useful for industrial researches.
Keywords: Characterization; E. coli; Granulocyte Colony-Stimulating Factor.
Copyright© by Royan Institute. All rights reserved.
Conflict of interest statement
There is no conflict of interest in this study.
Figures

References
-
- Jin H, Cantin GT, Maki S, Chew LC, Resnick SM, Ngai J, et al. Soluble periplasmic production of human granulocyte colony-stimulating factor (G-CSF) in Pseudomonas fluorescens. Protein Expr Purif. 2011;78(1):69–77. - PubMed
-
- Dale D. Current management of chemotherapy-induced neutropenia: the role of colony-stimulating factors. Semin Oncol. 2003;30(4 Suppl 13):3–9. - PubMed
-
- Nishii K, Xing XH, Shiragami N, Unno H. Production of rG-CSF by CHO cell in aggregate microbeads culture. Cytotechnol. 1994;6(7):435–439.
-
- Frampton JE, Yarker YE, Goa KL. Lenograstim.A review of its pharmacological properties and therapeutic efficacy in neutropenia and related clinical settings. Drugs. 1995;49(5):767–793. - PubMed
-
- Souza L M, Boone T C, Gabrilove J, Lai P H, Zsebo K M, Murdock D C, et al. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986;232(4746):61–65. - PubMed
LinkOut - more resources
Full Text Sources
