The Neuroprotective Effect of Steroid Receptor Coactivator-Interacting Protein (SIP) in Astrocyte Model of 1-Methyl-4-Phenylpyridinium (MPP⁺)-Induced Parkinson's Disease
- PMID: 31376345
- PMCID: PMC6690214
- DOI: 10.12659/MSM.912106
The Neuroprotective Effect of Steroid Receptor Coactivator-Interacting Protein (SIP) in Astrocyte Model of 1-Methyl-4-Phenylpyridinium (MPP⁺)-Induced Parkinson's Disease
Abstract
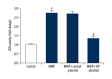
BACKGROUND The purpose of this study was to investigate the role and mechanism of steroid receptor coactivator-interacting protein (SIP) in an astrocyte model of 1-methyl-4-phenylpyridinium (MPP⁺)-induced Parkinson's disease. MATERIAL AND METHODS To perform our study, a Parkinson's disease cell model was established by treating the rat glioblastoma cell line C6 with MPP⁺. SIP was overexpressed in C6 cells using SIP-plasmid. Cell viability and apoptosis were analyzed using MTT assay and flow cytometer respectively. Tumor necrosis factor (TNF)-alpha and interleukin (IL)-1ß levels were detected using enzyme linked immunosorbent assay and quantitative reverse transcription PCR. Besides, lactate dehydrogenase (LDH) release, reactive oxygen species (ROS) production, and superoxide dismutase (SOD) enzyme activity were determined in the present study. For protein and mRNA detection, western blot assay, and qRT-PCR were performed respectively. RESULTS SIP was decreased in MPP⁺-induced C6 cells. SIP overexpression relieved MPP⁺-induced cytotoxicity of C6 cells, displayed as increased cell viability and reduced cell apoptosis and reduced LDH release. Besides, SIP inhibited MPP⁺-induced inflammatory response and oxidative stress, evidenced by decreased levels of inflammatory factors (TNF-alpha and IL-1ß), reduced ROS generation and enhanced SOD activity. Moreover, MPP⁺-induced nuclear factor-kappaB activation was inhibited by SIP overexpression. CONCLUSIONS SIP was downregulated in Parkinson's disease and it played a protective role in the development Parkinson's disease, thus may be a promising target for Parkinson's disease treatment.
Conflict of interest statement
None.
Figures






References
-
- Katunina EA, Titova NV, Malykhina EA, et al. [Oxidative stress and Parkinson’s disease: Mechanisms and perspectives of treatment]. Zh Nevrol Psikhiatr Im S S Korsakova. 2015;115:141–45. [in Russian] - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

