Mechanisms of intergenerational transmission of polycystic ovary syndrome
- PMID: 31376813
- PMCID: PMC6989388
- DOI: 10.1530/REP-19-0197
Mechanisms of intergenerational transmission of polycystic ovary syndrome
Abstract
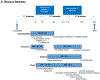
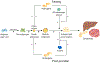
Developmental origins of adult disease (DoHAD) refers to critical gestational ages during human fetal development and beyond when the endocrine metabolic status of the mother can permanently program the physiology and/or morphology of the fetus, modifying its susceptibility to disease after birth. The aim of this review is to address how DoHAD plays an important role in the phenotypic expression of polycystic ovary syndrome (PCOS), the most common endocrinopathy of women characterized by hyperandrogenism, oligo-anovulation and polycystic ovarian morphology. Clinical studies of PCOS women are integrated with findings from relevant animal models to show how intergenerational transmission of these central components of PCOS are programmed through an altered maternal endocrine-metabolic environment that adversely affects the female fetus and long-term offspring health. Prenatal testosterone treatment in monkeys and sheep have been particularly crucial in our understanding of developmental programming of PCOS because organ system differentiation in these species, as in humans, occurs during fetal life. These animal models, along with altricial rodents, produce permanent PCOS-like phenotypes variably characterized by LH hypersecretion from reduced steroid-negative feedback, hyperandrogenism, ovulatory dysfunction, increased adiposity, impaired glucose-insulin homeostasis and other metabolic abnormalities. The review concludes that DoHAD underlies the phenotypic expression of PCOS through an altered maternal endocrine-metabolic environment that can induce epigenetic modifications of fetal genetic susceptibility to PCOS after birth. It calls for improved maternal endocrine-metabolic health of PCOS women to lower their risks of pregnancy-related complications and to potentially reduce intergenerational susceptibility to PCOS and its metabolic derangements in offspring.
Conflict of interest statement
Figures


References
-
- ABBOTT DH, BARNETT DK, BRUNS CM & DUMESIC DA 2005. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update, 11, 357–74. - PubMed
-
- ABBOTT DH, BRUNS CM, BARNETT DK & DUMESIC DA 2007. Fetal programming of polycystic ovary syndrome In: KOVACS G & NORMAN R (eds.) Polycystic Ovary Syndrome. 2nd ed Cambridge: Cambridge University Press.
-
- ABBOTT DH, DUMESIC DA, EISNER JE, KEMNITZ JW & GOY RW 1997. The Prenatally Androgenized Female Rhesus Monkey as a Model for PCOS In: NESTLER JE, DEWAILLY D & AZZIZ R (eds.) Androgen Excess Disorders in Women. Phyladelphia, Pennsylvannia: Lippincott-Raven Press.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

