Lysosome imaging in cancer cells by pyrene-benzothiazolium dyes: An alternative imaging approach for LAMP-1 expression based visualization methods to avoid background interference
- PMID: 31377388
- PMCID: PMC7065667
- DOI: 10.1016/j.bioorg.2019.103144
Lysosome imaging in cancer cells by pyrene-benzothiazolium dyes: An alternative imaging approach for LAMP-1 expression based visualization methods to avoid background interference
Abstract
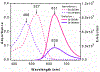
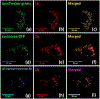
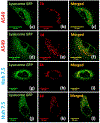
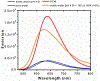
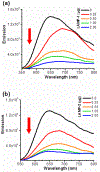
A series of pyrene-benzothiazolium dyes (1a-1d) were experimentally investigated to study their internalization mechanism into cellular lysosomes as well as their potential imaging applications for live cell imaging. The lysosome selectivity of the probes was further compared by using fluorescently tagged lysosome associated membrane protein-1 (LAMP-1) expression-dependent visualization in both normal (COS-7, HEK293) and cancer (A549, Huh 7.5) cell lines. These probes were successfully employed as reliable lysosome markers in tumor cell models, thus providing an attractive alternative to LAMP-1 expression-dependent visualization methods. One advantage of these probes is the elimination of significant background fluorescence arising from fluorescently tagged protein expression on the cell surface when cells were transfected with LAMP-1 expression plasmids. Probes exhibited remarkable ability to stain cellular lysosomes for long-term experiments (up to 24 h) and the highly lipophilic nature of the probe design allowed their accumulation in hydrophobic regions of the cellular lysosomes. Experimental evidences indicated that the probes are likely to be internalized into lysosomes via endocytosis and accumulated in the hydrophobic regions of the lysosomes rather than in the acidic lysosomal lumen. These probes also demonstrated significant stability and lysosome staining for fixed cell imaging applications as well. Lastly, the benzothiazolium moiety of the probes was identified as the key component for lysosome selectivity.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing Interests
Authors declare no competing interests.
Figures






References
-
- Parkinson-Lawrence EJ, Shandala T, Prodoehl M, Plew R, Borlace GN, Brooks DA, Lysosomal storage disease: revealing lysosomal function and physiology, Physiology. 25 (2010)102–115. - PubMed
-
- Kirkegaard T, Jäättelä M, Lysosomal involvement in cell death and cancer, Biochim. Biophys. Acta - Mol. Cell Res 1793 (2009) 746–754. - PubMed
-
- Maes H, Agostinis P, Autophagy and mitophagy interplay in melanoma progression, Mitochondrion. (2014) 58–68. - PubMed
-
- Honscheid P, Datta K, Muders MH, Autophagy: detection, regulation and its role in cancer and therapy response, Int J Radiat Biol. 90 (2014) 628–635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

