Mucosal microbial parasites/symbionts in health and disease: an integrative overview
- PMID: 31378213
- PMCID: PMC6817359
- DOI: 10.1017/S0031182019000647
Mucosal microbial parasites/symbionts in health and disease: an integrative overview
Abstract
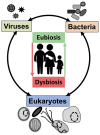
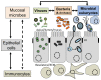
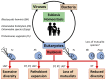
Microbial parasites adapted to thrive at mammalian mucosal surfaces have evolved multiple times from phylogenetically distant lineages into various extracellular and intracellular life styles. Their symbiotic relationships can range from commensalism to parasitism and more recently some host-parasites interactions are thought to have evolved into mutualistic associations too. It is increasingly appreciated that this diversity of symbiotic outcomes is the product of a complex network of parasites-microbiota-host interactions. Refinement and broader use of DNA based detection techniques are providing increasing evidence of how common some mucosal microbial parasites are and their host range, with some species being able to swap hosts, including from farm and pet animals to humans. A selection of examples will illustrate the zoonotic potential for a number of microbial parasites and how some species can be either disruptive or beneficial nodes in the complex networks of host-microbe interactions disrupting or maintaining mucosal homoeostasis. It will be argued that mucosal microbial parasitic diversity will represent an important resource to help us dissect through comparative studies the role of host-microbe interactions in both human health and disease.
Keywords: Bacteria; extracellular and intracellular parasites; innate and adaptive immune responses; microbiota; mucosa; parasitic protists; parasitic protozoa; pathobionts; viruses.
Figures
Similar articles
-
Unique characteristics of local responses in host resistance to mucosal parasitic infections.Vet Parasitol. 1986 Mar;20(1-3):175-94. doi: 10.1016/0304-4017(86)90099-3. Vet Parasitol. 1986. PMID: 3518214 Review.
-
Host-symbiont-pathogen interactions in blood-feeding parasites: nutrition, immune cross-talk and gene exchange.Parasitology. 2018 Sep;145(10):1294-1303. doi: 10.1017/S0031182018000574. Epub 2018 Apr 12. Parasitology. 2018. PMID: 29642965 Review.
-
Chronic infections with viruses or parasites: breaking bad to make good.Immunology. 2017 Apr;150(4):389-396. doi: 10.1111/imm.12703. Epub 2017 Jan 19. Immunology. 2017. PMID: 28009488 Free PMC article. Review.
-
Factors involved in symbiosis and host resistance at the mucosa-parasite interface.Prog Allergy. 1982;31:76-177. Prog Allergy. 1982. PMID: 6183674 Review. No abstract available.
-
The role of defensive symbionts in host-parasite coevolution.Biol Rev Camb Philos Soc. 2018 Nov;93(4):1747-1764. doi: 10.1111/brv.12417. Epub 2018 Apr 16. Biol Rev Camb Philos Soc. 2018. PMID: 29663622 Review.
Cited by
-
Risk of Secondary Bacterial Infections Revealed by Changes in Trachinotus ovatus Skin and Gill Microbiota During a Cryptocaryon irritans Infection Cycle.Microorganisms. 2025 Jul 14;13(7):1660. doi: 10.3390/microorganisms13071660. Microorganisms. 2025. PMID: 40732169 Free PMC article.
-
Fecal microbiota transplantation from protozoa-exposed donors downregulates immune response in a germ-free mouse model, its role in immune response and physiology of the intestine.PLoS One. 2024 Oct 28;19(10):e0312775. doi: 10.1371/journal.pone.0312775. eCollection 2024. PLoS One. 2024. PMID: 39466773 Free PMC article.
-
Clinical implications of trichomonads detected in bronchoalveolar fluid by metagenomic next-generation sequencing: a multicenter retrospective study.Front Cell Infect Microbiol. 2024 Jan 22;14:1289231. doi: 10.3389/fcimb.2024.1289231. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38318165 Free PMC article.
-
Diagnostic Methods of Common Intestinal Protozoa: Current and Future Immunological and Molecular Methods.Trop Med Infect Dis. 2022 Sep 21;7(10):253. doi: 10.3390/tropicalmed7100253. Trop Med Infect Dis. 2022. PMID: 36287994 Free PMC article. Review.
-
Shifts in the Fecal Microbial Community of Cystoisospora suis Infected Piglets in Response to Toltrazuril.Front Microbiol. 2020 May 19;11:983. doi: 10.3389/fmicb.2020.00983. eCollection 2020. Front Microbiol. 2020. PMID: 32508791 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

