Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: an open-label, randomised controlled trial
- PMID: 31378394
- PMCID: PMC6857437
- DOI: 10.1016/S0140-6736(19)31282-6
Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: an open-label, randomised controlled trial
Abstract
Background: Hypertension is the most common medical disorder in pregnancy, complicating one in ten pregnancies. Treatment of severely increased blood pressure is widely recommended to reduce the risk for maternal complications. Regimens for the acute treatment of severe hypertension typically include intravenous medications. Although effective, these drugs require venous access and careful fetal monitoring and might not be feasible in busy or low-resource environments. We therefore aimed to compare the efficacy and safety of three oral drugs, labetalol, nifedipine retard, and methyldopa for the management of severe hypertension in pregnancy.
Methods: In this multicentre, parallel-group, open-label, randomised controlled trial, we compared these oral antihypertensives in two public hospitals in Nagpur, India. Pregnant women were eligible for the trial if they were aged at least 18 years; they were pregnant with fetuses that had reached a gestational age of at least 28 weeks; they required pharmacological blood pressure control for severe hypertension (systolic blood pressure ≥160 mm Hg or diastolic blood pressure ≥110 mm Hg); and were able to swallow oral medications. Women were randomly assigned to receive 10 mg oral nifedipine, 200 mg oral labetalol (hourly, in both of which the dose could be escalated if hypertension was maintained), or 1000 mg methyldopa (a single dose, without dose escalation). Masking of participants, study investigators, and care providers to group allocation was not possible because of different escalation protocols in the study groups. The primary outcome was blood pressure control (defined as 120-150 mm Hg systolic blood pressure and 70-100 mm Hg diastolic blood pressure) within 6 h with no adverse outcomes. This study is registered with ClinicalTrials.gov, number NCT01912677, and the Clinical Trial Registry, India, number ctri/2013/08/003866.
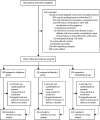
Findings: Between April 1, 2015, and Aug 21, 2017, we screened 2307 women for their inclusion in the study. We excluded 1413 (61%) women who were ineligible, declined to participate, had impending eclampsia, were in active labour, or had a combination of these factors. 11 (4%) women in the nifedipine group, ten (3%) women in the labetalol group, and 11 (4%) women in the methyldopa group were ineligible for treatment (because they had only one qualifying blood pressure measurement) or had treatment stopped (because of delivery or transfer elsewhere). 894 (39%) women were randomly assigned to a treatment group and were included in the intention-to-treat analysis: 298 (33%) women were assigned to receive nifedipine, 295 (33%) women were assigned to receive labetalol, and 301 (33%) women were assigned to receive methyldopa. The primary outcome was significantly more common in women in the nifedipine group than in those in the methyldopa group (249 [84%] women vs 230 [76%] women; p=0·03). However, the primary outcome did not differ between the nifedipine and labetalol groups (249 [84%] women vs 228 [77%] women; p=0·05) or the labetalol and methyldopa groups (p=0·80). Seven serious adverse events (1% of births) were reported during the study: one (<1%) woman in the labetalol group had an intrapartum seizure and six (1%) neonates (one [<1%] neonate in the nifedipine group, two [1%] neonates in the labetalol group, and three [1%] neonates in the methyldopa group) were stillborn. No birth had more than one adverse event.
Interpretation: All oral antihypertensives reduced blood pressure to the reference range in most women. As single drugs, nifedipine retard use resulted in a greater frequency of primary outcome attainment than labetalol or methyldopa use. All three oral drugs-methyldopa, nifedipine, and labetalol-are viable initial options for treating severe hypertension in low-resource settings.
Funding: PREEMPT (University of British Columbia, Vancouver, BC, Canada; grantee of Bill & Melinda Gates Foundation).
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Oral regimen management of acute hypertension in pregnancy.Lancet. 2019 Sep 21;394(10203):981-982. doi: 10.1016/S0140-6736(19)31717-9. Epub 2019 Aug 1. Lancet. 2019. PMID: 31378396 No abstract available.
-
Contemporary Issues in Women's Health.Int J Gynaecol Obstet. 2020 Jan;148(1):3-5. doi: 10.1002/ijgo.13026. Epub 2019 Nov 17. Int J Gynaecol Obstet. 2020. PMID: 31736061 No abstract available.
References
-
- Brown MA, Magee LA, Kenny LC. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310. - PubMed
-
- Souza JP, Gülmezoglu AM, Vogel J. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet. 2013;381:1747–1755. - PubMed
-
- Butalia S, Audibert F, Côté AM. Hypertension Canada's 2018 guidelines for the management of hypertension in pregnancy. Can J Cardiol. 2018;34:526–531. - PubMed
-
- Committee on Obstetric Practice Committee opinion no. 692: emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;129:e90–e95. - PubMed
-
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36:416–441. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

