Inhibition of Axon Regeneration by Liquid-like TIAR-2 Granules
- PMID: 31378567
- PMCID: PMC6813885
- DOI: 10.1016/j.neuron.2019.07.004
Inhibition of Axon Regeneration by Liquid-like TIAR-2 Granules
Abstract
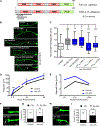
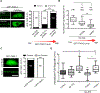
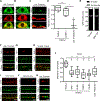
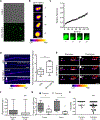
Phase separation into liquid-like compartments is an emerging property of proteins containing prion-like domains (PrLDs), yet the in vivo roles of phase separation remain poorly understood. TIA proteins contain a C-terminal PrLD, and mutations in the PrLD are associated with several diseases. Here, we show that the C. elegans TIAR-2/TIA protein functions cell autonomously to inhibit axon regeneration. TIAR-2 undergoes liquid-liquid phase separation in vitro and forms granules with liquid-like properties in vivo. Axon injury induces a transient increase in TIAR-2 granule number. The PrLD is necessary and sufficient for granule formation and inhibiting regeneration. Tyrosine residues within the PrLD are important for granule formation and inhibition of regeneration. TIAR-2 is also serine phosphorylated in vivo. Non-phosphorylatable TIAR-2 variants do not form granules and are unable to inhibit axon regeneration. Our data demonstrate an in vivo function for phase-separated TIAR-2 and identify features critical for its function in axon regeneration.
Keywords: C. elegans; LLPS; RNA granule; RNA-binding protein; TIA1; axon injury; axon regeneration; liquid-liquid phase separation; prion-like domain; stress granule; tiar-2.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interest.
Figures


Comment in
-
Axons Gonna Ride 'til They Can't No More.Neuron. 2019 Oct 23;104(2):179-181. doi: 10.1016/j.neuron.2019.09.029. Neuron. 2019. PMID: 31647889
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

