Male Sterility in Maize after Transient Heat Stress during the Tetrad Stage of Pollen Development
- PMID: 31378720
- PMCID: PMC6776839
- DOI: 10.1104/pp.19.00707
Male Sterility in Maize after Transient Heat Stress during the Tetrad Stage of Pollen Development
Abstract
Shifts in the duration and intensity of ambient temperature impair plant development and reproduction, particularly male gametogenesis. Stress exposure causes meiotic defects or premature spore abortion in male reproductive organs, leading to male sterility. However, little is known about the mechanisms underlying stress and male sterility. To elucidate these mechanisms, we imposed a moderate transient heat stress on maize (Zea mays) plants at the tetrad stage of pollen development. After completion of pollen development at optimal conditions, stress responses were assessed in mature pollen. Transient heat stress resulted in reduced starch content, decreased enzymatic activity, and reduced pollen germination, resulting in sterility. A transcriptomic comparison pointed toward misregulation of starch, lipid, and energy biosynthesis-related genes. Metabolomic studies showed an increase of Suc and its monosaccharide components, as well as a reduction in pyruvate. Lipidomic analysis showed increased levels of unsaturated fatty acids and decreased levels of saturated fatty acids. In contrast, the majority of genes involved in developmental processes such as those required for auxin and unfolded protein responses, signaling, and cell wall biosynthesis remained unaltered. It is noteworthy that changes in the regulation of transcriptional and metabolic pathway genes, as well as heat stress proteins, remained altered even though pollen could recover during further development at optimal conditions. In conclusion, our findings demonstrate that a short moderate heat stress during the highly susceptible tetrad stage strongly affects basic metabolic pathways and thus generates germination-defective pollen, ultimately leading to severe yield losses in maize.
© 2019 American Society of Plant Biologists. All Rights Reserved.
Figures
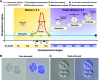


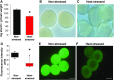



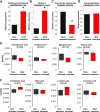


Comment in
-
The Heat Is On: Maize Pollen Development after a Heat Wave.Plant Physiol. 2019 Oct;181(2):387-388. doi: 10.1104/pp.19.01025. Plant Physiol. 2019. PMID: 31582472 Free PMC article. No abstract available.
References
-
- Alghabari F, Lukac M, Jones HE, Gooding MJ (2014) Effect of Rht alleles on the tolerance of wheat grain set to high temperature and drought stress during booting and anthesis. J Agron Crop Sci 200: 36–45
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

